Mechanism of mTOR/RILP-regulated autophagic flux in increased susceptibility to myocardial ischemia-reperfusion in diabetic mice
- PMID: 39958873
- PMCID: PMC11825452
- DOI: 10.3389/fphar.2024.1506401
Mechanism of mTOR/RILP-regulated autophagic flux in increased susceptibility to myocardial ischemia-reperfusion in diabetic mice
Abstract
Background: The increased myocardial vulnerability that occurs in diabetic patients following an ischemia-reperfusion injury (I/RI) represents a significant perioperative safety risk. A comprehensive understanding of the intrinsic mechanisms underlying this phenomenon is therefore of paramount importance.
Purposes: The objective of this study is to investigate the potential mechanism of action between impaired autophagic flux and increased vulnerability in diabetic myocardium. This will provide a foundation for the clinical search for effective preventive and curative measures.
Methods: The transcriptomic alterations in autophagy-related genes following myocardial exposure to I/RI were analyzed by single-cell sequencing. This was followed by the validation of potential mechanisms of action between impaired autophagic flux and increased susceptibility at the cellular and animal levels, respectively.
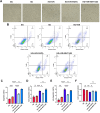
Results: After I/RI in diabetic myocardium, there was a significant increase in the number of CM1 subgroups and a specific downregulation of 239 autophagy-related genes led by RILP. HE staining revealed that myocardial injury was exacerbated in diabetic mice subjected to I/RI. Transmission electron microscopy revealed that the accumulation of autophagic vesicles in cardiomyocytes of diabetic mice resulted in impaired autophagic flux. qRT-PCR revealed that the expression of RILP was significantly reduced in diabetic mice subjected to I/RI. WB showed that P62 was significantly increased and RILP was significantly decreased in diabetic mice subjected to I/RI compared to healthy mice. Inhibition of mTOR during hypoxia/reoxygenation (H/R) injury restored RILP expression and attenuated cellular injury in cardiomyocytes cultured with high glucose.
Conclusion: Following I/RI in diabetic myocardium, an increase in the CM1 subpopulation and a reduction in RILP expression result in impaired autophagic flux. Regulation of the mTOR/RILP pathway can restore impaired autophagic flux and improve myocardial vulnerability, thereby exerting cardioprotective effects.
Keywords: autophagic flux; diabetic cardiomyopathy; mTOR/RILP pathway; myocardial protection; vulnerability.
Copyright © 2025 Zhao, Shi, Zheng, Wang, Yuan, Anwar, He, Ma and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

