Quantification of Antiretroviral Drug Emtricitabine in Human Plasma by Surface Enhanced Raman Spectroscopy
- PMID: 39959057
- PMCID: PMC11822520
- DOI: 10.1021/acsomega.4c06162
Quantification of Antiretroviral Drug Emtricitabine in Human Plasma by Surface Enhanced Raman Spectroscopy
Abstract
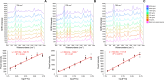
In this study, reproducible label-free detection and quantification of the antiretroviral drug emtricitabine (FTC) down to 78 ng/mL in human plasma by surface enhanced Raman spectroscopy (SERS) is presented. A novel plasma sample pretreatment method using silver nitrate and silver colloidal nanoparticles (Ag CNPs) was used to prepare the plasma samples for analysis. The pretreated plasma samples were evaporated to dryness on an aluminum surface and a computer-controlled Raman scanning system was used to collect spatially resolved SERS spectra of the entire surface. Calibration curves of commercial human plasma samples containing FTC in a concentration range of 5000 to 78 ng/mL were calculated using three different methods. First, a conventional approach was taken, where all the spectra collected for each concentration were averaged, then the SERS intensity of a known FTC peak (792 cm-1) was used for calibrations (total population method). This approach was refined by utilizing a figure-of-merit (FOM) quality index (Q i) to sample spectra from each concentration that contained the highest signal-to-noise (S/N), before averaging and calculating the SERS intensity of the 792 cm-1 FTC peak (Q i sample method). Finally, the distribution of all Q i values for each concentration were modeled using cumulative distribution functions (CDFs) and were used for calibrations (CDF method). The CDF method exhibited the highest analytical sensitivity (slope = 3702.47) compared to the Q i sample method (slope = 1591.05) and the total population method (slope = 754.21). The Q i sample method exhibited the highest linearity (R 2 = 0.99) compared to the CDF method (R 2 = 0.95) and the total population average (R 2 = 0.97). The CDF method exhibited the highest S/N in the concentration range of 5000 to 312 ng/mL (S/N range of 31.5-16.6). The Q i sample method exhibited the highest S/N for concentrations 156 and 78 ng/mL (S/N = 9.7 and 7.4, respectively). These results show that the Q i sample method is advantageous over all other methods when approaching the LOQ while the CDF method is advantageous over all methods at higher concentrations. The LOQ (78 ng/mL) was confirmed by principal component analysis (PCA). Together these results show that statistical treatment of a large population of SERS spectra, where the analyte signal intensity follows an exponential distribution, is superior to standard methods of averaging populations of spectra in terms of analytical sensitivity, linearity, and S/N. Additionally, it was found that the background signal had no interference with the quantitative data calculated for the total population and Q i sample methods after repeating both analyses with baseline-subtracted spectra. The results and methodology presented in this study establish a framework for integrating SERS into drug adherence monitoring for FTC-based treatment and prevention of infections by demonstrating consistent SERS detection and quantification of FTC in human plasma at therapeutically relevant concentrations.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Schaecher K. L. The importance of treatment adherence in HIV. Am. J. Manag. Care 2013, 19 (12 Suppl), s231–s237. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
