High-Mobility Electrons in Aqueous Iodide Solutions
- PMID: 39959103
- PMCID: PMC11822714
- DOI: 10.1021/acsomega.4c11040
High-Mobility Electrons in Aqueous Iodide Solutions
Abstract
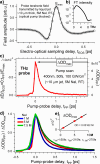
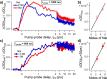
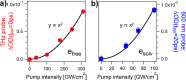
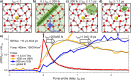
The photoexcitation of aqueous iodide solutions is a prototype for the generation of electrons in liquid water. Upon one-photon excitation, the precursors of the solvated electrons are localized states with a radius of a few angstroms. In contrast, with the aid of transient absorption spectroscopy at terahertz, near-infrared, and visible frequencies, we show that the two-photon absorption of ∼400 nm pulses can impulsively generate short-lived (∼250 fs), delocalized electrons that are released tens of angstroms away from the parent ion. We propose that these states can be ascribed to 5p → 6p transitions that, in turn, could be thought of as frustrated Rydberg orbitals or large radius excitons. By capitalizing on the unique capabilities of transient terahertz spectroscopy, we estimate that these delocalized states are characterized by an electronic mobility and diffusivity that are about 500 times greater than those of the fully relaxed electrons.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sheu W. S.; Rossky P. J. Dynamics of Electron Photodetachment from an Aqueous Halide Ion. Chem. Phys. Lett. 1993, 213 (3–4), 233–238. 10.1016/0009-2614(93)85125-8. - DOI
-
- Bradforth S. E.; Jungwirth P. Excited States of Iodide Anions in Water: A Comparison of the Electronic Structure in Clusters and in Bulk Solution. J. Phys. Chem. A 2002, 106 (7), 1286–1298. 10.1021/jp013068p. - DOI
LinkOut - more resources
Full Text Sources
