Advanced One-Pot RPA-CRISPR/Cas12a Reaction with Glycerol and Betaine for High-Sensitivity Diagnosis of mecA-Carrying Strains in Clinical Samples
- PMID: 39959114
- PMCID: PMC11822479
- DOI: 10.1021/acsomega.4c09078
Advanced One-Pot RPA-CRISPR/Cas12a Reaction with Glycerol and Betaine for High-Sensitivity Diagnosis of mecA-Carrying Strains in Clinical Samples
Abstract
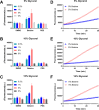
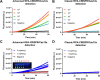
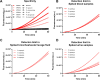
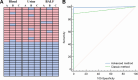
The mecA gene confers methicillin resistance in both MRSA and MR-CoNS by encoding the PBP2a protein and poses a significant public health threat due to its resistance to beta-lactam antibiotics. Rapid and accurate detection of mecA is critical for timely treatment, reducing morbidity, and preventing its spread in healthcare settings. In this study, we developed an advanced one-pot recombinase polymerase amplification (RPA)-CRISPR/Cas12a system, enhanced with glycerol and betaine, for ultrasensitive detection of the mecA gene. Glycerol's viscosity effect prevents premature interaction between Cas12a and early amplification products, while betaine enhances nucleic acid amplification. The assay demonstrated superior sensitivity, detecting as low as 5 copies/μL of mecA DNA within 60 min. Specificity testing against a panel of bacterial species confirmed the high selectivity of the assay for mecA-carrying strains with negligible cross-reactivity. Furthermore, this method exhibited excellent performance across various clinical samples, including blood, urine, and bronchoalveolar lavage fluid. Our findings underscore the potential of this advanced RPA-CRISPR/Cas12a assay as a powerful diagnostic tool for rapid, cost-effective, and highly sensitive mecA detection, offering a promising solution for clinical diagnostics and infection control.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Cas12a/Guide RNA-Based Platforms for Rapidly and Accurately Identifying Staphylococcus aureus and Methicillin-Resistant S. aureus.Microbiol Spectr. 2023 Mar 21;11(2):e0487022. doi: 10.1128/spectrum.04870-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36943040 Free PMC article.
-
A rapid, specific and ultrasensitive detection of the Chikungunya virus based on RT-RPA:CRISPR/Cas12a one-pot dual mode end-point detection system.Anal Chim Acta. 2024 Nov 15;1329:343221. doi: 10.1016/j.aca.2024.343221. Epub 2024 Sep 11. Anal Chim Acta. 2024. PMID: 39396286
-
A photocontrolled one-pot isothermal amplification and CRISPR-Cas12a assay for rapid detection of SARS-CoV-2 Omicron variants.Microbiol Spectr. 2024 Mar 5;12(3):e0364523. doi: 10.1128/spectrum.03645-23. Epub 2024 Feb 6. Microbiol Spectr. 2024. PMID: 38319081 Free PMC article.
-
One-pot platform for rapid detecting virus utilizing recombinase polymerase amplification and CRISPR/Cas12a.Appl Microbiol Biotechnol. 2022 Jun;106(12):4607-4616. doi: 10.1007/s00253-022-12015-9. Epub 2022 Jun 16. Appl Microbiol Biotechnol. 2022. PMID: 35708748 Free PMC article.
-
KP177R-based visual assay integrating RPA and CRISPR/Cas12a for the detection of African swine fever virus.Front Immunol. 2024 Apr 9;15:1358960. doi: 10.3389/fimmu.2024.1358960. eCollection 2024. Front Immunol. 2024. PMID: 38655256 Free PMC article.
References
LinkOut - more resources
Full Text Sources
