Analysis of Smad3 in the modulation of stromal extracellular matrix proteins in corneal scarring after alkali injury
- PMID: 39959170
- PMCID: PMC11829792
Analysis of Smad3 in the modulation of stromal extracellular matrix proteins in corneal scarring after alkali injury
Abstract
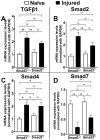
Purpose: During ocular trauma, excessive proliferation and transdifferentiation of corneal stromal fibroblasts cause haze/fibrosis in the cornea. Transforming growth factor β (TGFβ) plays a key role in corneal fibrosis through the Smad signaling pathway. The aberrant activity of TGFβ signaling during ocular trauma (viz. mechanical, infectious, chemical, or surgically altered TGFβ/Smad signaling) leads to regulating the predominant expression of myogenic proteins and the extracellular matrix (ECM). We sought to investigate the functional role of Smad3 in corneal wound repair and stromal ECM assembly using Smad3+/+ wild-type and Smad3-/- deficient mice.
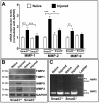
Methods: Corneal injury was introduced with the topical application of an alkali-soaked 2-mm filter disc on the central cornea in the Smad3+/+ (C57BL/6J) and Smad3-/- (129-Smad3tm1Par/J) mouse strains. Slit-lamp and stereo microscopy were used for clinical assessment and corneal haze grading in live animals. Hematoxylin and eosin and Masson's trichrome staining were used to study comparative morphology and collagen level alterations between the groups. Real-time qRT-PCR, western blot, and immunohistochemistry were used to measure changes in profibrotic genes at the mRNA and protein levels.
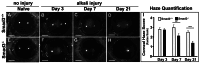
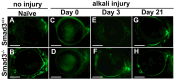
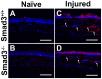
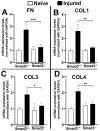
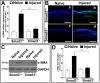
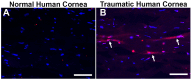
Results: Slit-lamp clinical exams and stereo microscopy detected notably less opaque cornea in the eyes of Smad3-/- compared with Smad3+/+ mice at 3 weeks (p<0.01) in live animals. Corneal tissue sections of Smad3-/- mice showed significantly fewer α-smooth muscle actin-positive cells compared with those of the Smad3+/+ animals (p<0.05). The corneas of the Smad3-/- mice showed significantly lower mRNA levels of pro-fibrotic genes, α-smooth muscle actin, fibronectin, and collagen I (p<0.05, p<0.01, and p<0.001). In addition, the matrix metalloproteinase and tissue inhibitors of metalloproteinase levels were significantly increased (p<0.001) in the corneal tissue during alkali injury in both Smad3+/+ wild-type and Smad3-/- deficient mice.
Conclusions: The significant changes in profibrotic genes and stromal ECM proteins revealed a direct role of Smad3 in stromal ECM proteins and TGFβ/Smad-driven wound healing. Smad3 appears to be an attractive molecular target for limiting abnormal stroma wound healing to treat corneal fibrosis in vivo.
Copyright © 2024 Molecular Vision.
Figures

Similar articles
-
Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo.Exp Eye Res. 2022 Mar;216:108933. doi: 10.1016/j.exer.2022.108933. Epub 2022 Jan 11. Exp Eye Res. 2022. PMID: 35031282 Free PMC article.
-
Stromal fibroblasts are associated with collagen IV in scar tissues of alkali-burned and lacerated corneas.Curr Eye Res. 1997 Apr;16(4):339-48. doi: 10.1076/ceyr.16.4.339.10684. Curr Eye Res. 1997. PMID: 9134323
-
Characterization of C-X-C chemokine receptor type 5 in the cornea and role in the inflammatory response after corneal injury.Exp Eye Res. 2023 Jan;226:109312. doi: 10.1016/j.exer.2022.109312. Epub 2022 Nov 16. Exp Eye Res. 2023. PMID: 36400287 Free PMC article.
-
The corneal fibrosis response to epithelial-stromal injury.Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review.
-
Corneal Opacity: Cell Biological Determinants of the Transition From Transparency to Transient Haze to Scarring Fibrosis, and Resolution, After Injury.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):22. doi: 10.1167/iovs.63.1.22. Invest Ophthalmol Vis Sci. 2022. PMID: 35044454 Free PMC article. Review.
References
-
- Mohan RR, Gupta S, Kumar R, Sinha NR, Landreneau J, Sinha PR, Tandon A, Chaurasia SS, Hesemann NP. Tissue-targeted and localized AAV5-DCN and AAV5-PEDF combination gene therapy abrogates corneal fibrosis and concurrent neovascularization in rabbit eyes in vivo. Ocul Surf. 2024;32:13–25. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

