Genetic implications of CHST6 gene mutations and their corneal microstructural changes in macular corneal dystrophy patients
- PMID: 39959172
- PMCID: PMC11829787
Genetic implications of CHST6 gene mutations and their corneal microstructural changes in macular corneal dystrophy patients
Abstract
Purpose: To collectively investigate the carbohydrate sulfotransferase 6 (CHST6) mutation spectrum and corneal morphological alterations of macular corneal dystrophy (MCD) patients using in vivo confocal microscopy (IVCM), histochemistry, immunohistochemistry, and further ascertaining the immunophenotype using an enzyme-linked immunosorbent assay (ELISA).
Methods: Sanger sequencing-based CHST6 gene screening was performed for 112 study participants (MCD patients, n = 68; family members, n = 44). Twenty-seven MCD patients underwent IVCM analyses, and corneal buttons were analyzed with histochemistry Alcian blue (AB) staining and immunohistochemistry anti-keratan sulfate (KS) monoclonal antibody, 5D4MoAb. An ELISA was used to determine serum KS levels. Quantitative analysis of the central corneal thickness (CCT), epithelial cell thickness, epithelial cell count, and stromal keratocyte cell count was performed using a one-way ANOVA.
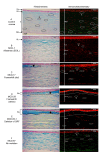
Results: Eighteen distinct CHST6 mutations, including one novel (p.L129V), were identified. MCD patients with predominant immunophenotype IA (n = 15) harboring major p.Q182Rfs199 deletion, p.194_R196delinsRC (delins), and open reading frame (ORF) mutations displayed AB positivity corresponding to loss of Bowman's layer, interlamellar glycosaminoglycan (GAG) depositions, and faint KS expression (5D4-MoAb) only in stromal keratocytes. Notably, IVCM imaging revealed BL loss due to confluent clumps of hyper-reflective, granular deposits together with scar tissue seen only in this group. Eight patients (with missense mutations) displayed immunophenotype I with positive GAG deposits and negative KS expression. Patients with immunophenotype II (n = 4) with no mutations showed both positive GAG deposits and KS expression. A quantitative analysis revealed a statistically significant decrease in CCT (p-value < 0.001), epithelial cell thickness, epithelial cell count, and stromal keratocyte cell count among the patients with truncation mutations compared to the control group.
Conclusions: In this current study, the combinational findings of MCD-related corneal morphological alterations, immunophenotypes, and mutation spectrum are presented first, which indicated a severe phenotype in patients identified with truncation (deletion, delins, and deletion of ORF) mutations. However, additional studies with a larger number of patients would help highlight these findings and reinforce the possible correlation between genotypes and immunophenotypes in MCD pathogenesis.
Copyright © 2024 Molecular Vision.
Figures




Similar articles
-
Immunophenotypes of macular corneal dystrophy in India and correlation with mutations in CHST6.Mol Vis. 2009;15:319-25. Epub 2009 Feb 9. Mol Vis. 2009. PMID: 19204788 Free PMC article.
-
Identification of novel mutations in the carbohydrate sulfotransferase gene (CHST6) causing macular corneal dystrophy.Invest Ophthalmol Vis Sci. 2002 Feb;43(2):377-82. Invest Ophthalmol Vis Sci. 2002. PMID: 11818380
-
Novel CHST6 gene mutations in 2 unrelated cases of macular corneal dystrophy.Cornea. 2011 Jun;30(6):664-9. doi: 10.1097/ICO.0b013e3182012888. Cornea. 2011. PMID: 21242781 Free PMC article.
-
[Immunophenotype classification of macular corneal dystrophy: first case report of immunophenotype I A outside of Saudi Arabia. A clinical histopathological correlation with immunohistochemistry and electron microscopy].Klin Monbl Augenheilkd. 2000 Aug;217(2):118-26. doi: 10.1055/s-2000-10395. Klin Monbl Augenheilkd. 2000. PMID: 11022667 Review. German.
-
Macular corneal dystrophy. Studies of sulfated glycosaminoglycans in corneal explant and confluent stromal cell cultures.Am J Pathol. 1977 Oct;89(1):167-82. Am J Pathol. 1977. PMID: 143892 Free PMC article. Review.
References
-
- Niel F, Ellies P, Dighiero P, Soria J, Sabbagh C, San C, Renard G, Delpech M, Valleix S. Truncating mutations in the carbohydrate sulfotransferase 6 gene (CHST6) result in macular corneal dystrophy. Invest Ophthalmol Vis Sci. 2003;44:2949–53. - PubMed
-
- Warren JF, Aldave AJ, Srinivasan M, Thonar EJ, Kumar AB, Cevallos V, Whitcher JP, Margolis TP. Novel mutations in the CHST6 gene associated with macular corneal dystrophy in southern India. Arch Ophthalmol. 2003;121:1608–12. - PubMed
-
- Murugan D, Prajna NV, Devi L, Sundaresan P. Genetic Analysis of CHST6 Gene in Indian Families with Macular Corneal Dystrophy. Int J Gen Sci.2017: 4:1–10. Surv Ophthalmol. 2018;63:609–17. - PubMed
-
- Aggarwal S, Peck T, Golen J, Karcioglu ZA. Macular corneal dystrophy: A review. Surv Ophthalmol. 2018;63:609–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

