Lefitolimod in Combination with Ipilimumab in Patients with Advanced Solid Tumors: A Phase I Trial
- PMID: 39959251
- PMCID: PMC11824599
- DOI: 10.36401/JIPO-24-17
Lefitolimod in Combination with Ipilimumab in Patients with Advanced Solid Tumors: A Phase I Trial
Abstract
Introduction: TLR9 agonists are immunomodulators that have been of interest for combined use with cancer immunotherapy. TLR9 agonists, such as lefitolimod (MGN1703), significantly increased Th1 response in preclinical models and have demonstrated efficacy in early clinical trials. This trial assessed the safety and preliminary efficacy of the combination of lefitolimod and ipilimumab in patients with advanced solid tumors.
Methods: This was a single-center, open-label, investigator-initiated phase I trial conducted at The University of Texas MD Anderson Cancer Center. Patients received leftolimod either subcutaneously (at escalating doses of 15-120 mg) or intratumorally (at the maximum feasible dose) in combination with ipilimumab (3 mg/kg). Paired biopsy samples were collected before the start of treatment and after two treatment cycles and analyzed by flow cytometry.
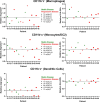
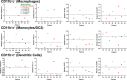
Results: We enrolled a total of 28 patients in this study with a median age of 56 years (range 19-75) in the escalation cohort and 60 years (range 34 -92) in the expansion cohort. The median number of prior lines of therapy was 4 (range 0-12). Eleven patients had at least one treatment-related adverse event (TRAE). The most common TRAEs were skin rash (n = 4, 14%), fatigue (n = 3, 11%), and pruritis (n = 2, 7%). No grade 4 or 5 AEs occurred, and no patients required dose reduction or treatment discontinuation due to AEs. The maximum tolerated dose (MTD) was not reached in this study. Of 28 patients, 21 patients had response-evaluable disease. No patients had a complete or partial response; 8 and 13 patients had stable and progressive disease as the best response, respectively. Paired biopsy samples were obtained from five patients. Increases in intratumoral CD8 T-cell frequency, memory CD8 phenotype (CD45RO+), and proliferation (Ki67+) in four of five patients suggested that the combination of lefitolimod and ipilimumab led to proinflammatory immune conditioning of the tumor microenvironment.
Conclusions: The combination of lefitolimod (administered subcutaneously or intratumorally) and ipilimumab was safe and well tolerated but demonstrated modest antitumor activity in patients with advanced cancers. ClinicalTrials.gov ID: NCT02668770.
Keywords: cancer therapeutics; checkpoint inhibitor; immunotherapy; intratumoral therapy; toll-like receptor.
Conflict of interest statement
Sources of Support: Supported in part by the NCI Cancer Center Support Grant P30CA016672 and Clinical and Translational Science Award Grant 1UM1 TR0045906 to The University of Texas MD Anderson Cancer Center.Conflict of Interest: Matthew J. Reilley, Sarina A. Piha-Paul, Siqing Fu, Apostolia M. Tsimberidou, Timothy A. Yap, Aung Naing, Jordi Rodon, Vivek Subbiah, Daniel Karp, Michael Curran, and David S. Hong have potential conflicts of interest listed at the end of the article. The remaining authors have no conflicts of interest.Matthew J. Reilley reports research funding from Adeli-Norte, Astrazeneca, Cardiff Oncology, Deciphera, Macrogenics, Merck, Pfizer, Seattle Genetics, Surface Oncology, Xencor, and ZielBio; and consulting/advisory role for Cardinal Health, Curio Science, Helsinn, Pfizer, and Seattle Genetics. Sarina A. Piha-Paul reports research funding (to institution) from AbbVie, ABM Therapeutics, Acepodia, Alkermes, Aminex Therapeutics, BioMarin Pharmaceutical, Boehringer Ingelheim, Bristol Myers Squib, Cerulean Pharma, Chugai Pharmaceutical Co., Curis, Cyclacel Pharmaceuticals, Daiichi Sankyo, Dohme, Eli Lilly, ENB Therapeutics, Epigenetix, Five Prime Therapeutics, F-Star Beta Limited, F-Star Therapeutics, Gene Quantum, Genmab A/S, Gilead Sciences, GlaxoSmithKline, Helix BioPharma, Hengrui Pharmaceuticals, HiberCell, Immunomedics, Incyte, Jacobio Pharmaceuticals, Jazz Pharmaceuticals, Jiangsu Simcere Pharmaceutical Co., Loxo Oncology, Lytix Biopharma AS, Medimmune, Medivation, Merck Sharp, Nectin Therapeutics, Novartis Pharmaceuticals, Nurix, OncoNano Medicine, Pieris Pharmaceuticals, Pfizer, Phanes Therapeutics, Principia Biopharma, ProFoundBio US, Puma Biotechnology, Purinomia Biotech, Rapt Therapeutics, Replimune; Roche/Blueprint, Seattle Genetics, Silverback Therapeutics, Shasqi, Synlogic Therapeutics, Taiho Oncology, Tallac Therapeutics, Tesaro, Theradex Oncology, Toragen Therapeutics, TransThera Bio, Xencor, ZielBio; and consulting for CRC Oncology and Lilly USA. Siqing Fu reports research support from Abbisko, Antengene, BeiGene, BeyongSpring Pharmaceuticals, BioAtla, Boehringer Ingelheim, CUE Biopharma, DEKA Biosciences, Eli Lilly & Co., Exelixis, Greenfire Bio, Hookipa Biotech, IMV, Innovent Biologics, Jazz Pharmaceuticlals, K-Group Beta, Lantern Pharma, Lyvgen Biopharm, MacroGenics, MediLink Therapeutics, Millennium Pharmaceuticals, Nerviano Medical Sciences, NeuPharma, NextCure, Ningbo NewBay Technology Development, Novartis, NovoCure, Nykode Therapeutics AS, Parexel International, PharmaMar USA, Pionyr Immunotherapeutics, PureTech Health, Qurgen, Shanghai Huaota Biopharmaceutical, Sellas Life Sciences Group, Soricimed Biopharma, SQZ Biotechnologies, Sumitomo Dainippon, Taiho Oncology, NCCN, Treadwell Therapeutics, Turnstone Biologics, Tyligand Bioscience, and Virogin Biotech. Apostolia M. Tsimberidou reports research support from 7 Hills Pharma, AbbVie, Agenus Bio, Cancer Prevention & Research Institute of Texas (CPRIT), Immatics Biotechnologies, Novocure, OBI Pharma, Orionis Biosciences, Parker Institute for Cancer Immunotherapy, Tachyon Therapeutics, Tempus Labs, Tvardi Therapeutics, and Vividion Therapeutics, consulting/advisory role for Vincerx, BrYet, Diaccurate, NEX-I, Macrogenics, and Avstera; and travel support from ASCO, Cancer Care at the Crossroads, Genome Web, and Precision Medicine World Conference. Timothy A. Yap reports research support from Artios, AstraZeneca, Bayer, Beigene, BioNTech, Blueprint, BMS, Boundless Bio, Clovis, Constellation, CPRIT, Cyteir, Department of Defense, Eli Lilly, EMD Serono, Exelixis, Forbius, F-Star, GlaxoSmithKline, Genentech, Gilead, Golfers against Cancer, Haihe, Ideaya, ImmuneSensor, Insilico Medicine, Ionis, Ipsen, Jounce, Karyopharm, KSQ, Kyowa, Merck, Mirati, Novartis, NIH/NCI, Pfizer, Pliant, Prelude, Ribon Therapeutics, Regeneron, Repare, Roche, Rubius, Sanofi, Scholar Rock, Seattle Genetics, Synnovation, Tango, Tesaro, V Foundation, Vivace, Zenith, and Zentalis; consulting for AbbVie, Acrivon, Adagene, Almac, Aduro, Amgen, Amphista, Artios, Astex, AstraZeneca, Athena, Atrin, Avenzo, Avoro, Axiom, Baptist Health Systems, Bayer, Beigene, BioCity Pharma, Blueprint, Boxer, BridGene Biosciences, Bristol Myers Squibb, C4 Therapeutics, Calithera, Cancer Research UK, Carrick Therapeutics, Circle Pharma, Clovis, Cybrexa, Daiichi Sankyo, Dark Blue Therapeutics, Debiopharm, Diffusion, Duke Street Bio, 858 Therapeutics, EcoR1 Capital, Ellipses Pharma, EMD Serono, Entos, FoRx Therapeutics AG, F-Star, Genesis Therapeutics, Genmab, Glenmark, GLG, Globe Life Sciences, Grey Wolf Therapeutics, GSK, Guidepoint, Ideaya Biosciences, Idience, Ignyta, I-Mab, ImmuneSensor, Impact Therapeutics, Institut Gustave Roussy, Intellisphere, Jansen, Joint Scientific Committee for Phase I Trials in Hong Kong, Kyn, Kyowa Kirin, MEI pharma, Mereo, Merck, Merit, Monte Rosa Therapeutics, Natera, Nested Therapeutics, Nexys, Nimbus, Novocure, Odyssey Therapeutics, OHSU, OncoSec, Ono Pharma, Onxeo, PanAngium Therapeutics, Pegascy, PER, Pfizer, Piper-Sandler, Pliant Therapeutics, Prelude Therapeutics, Prolynx, Protai Bio, Radiopharma Theranostics, Repare, resTORbio, Roche, Ryvu Therapeutics, SAKK, Sanofi, Schrodinger, Servier, Synnovation, Synthis Therapeutics, Tango, TCG Crossover, TD2, Terremoto Biosciences, Tessellate Bio, Theragnostics, Terns Pharmaceuticals, Thryv Therapeutics, Tolremo, Tome, Trevarx Biomedical, Varian, Veeva, Versant, Vibliome, Voronoi, Xinthera, Zai Labs, and ZielBio; and stock ownership in Seagen. Aung Naing reports research funding from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor/Millendo, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, Bristol-Myers Squibb, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Eli Lilly, Kymab, PsiOxus, Arcus Biosciences, NeoImmuneTech, Immune-Onc Therapeutics, Surface Oncology, Monopteros Therapeutics, BioNTech SE, Seven & Eight Biopharma, and SOTIO Biotech AG; consulting/advisory board role for CTI, Deka Biosciences, Janssen Biotech, NGM Bio, PsiOxus Therapeutics, Immune-Onc Therapeutics, STCube Pharmaceuticals, OncoSec KEYNOTE-695, Genome & Company, CytomX Therapeutics, Nouscom, Merck Sharp & Dohme Corp, Servier, Lynx Health, AbbVie, and PsiOxus; travel support from ARMO BioSciences, NeoImmuneTech, and NGM Biopharmaceuticals; honoraria from AKH Inc, The Lynx Group, Society for Immunotherapy of Cancer (SITC), Korean Society of Medical Oncology (KSMO), Scripps Cancer Care Symposium, ASCO Direct Oncology Highlights, European Society for Medical Oncology (ESMO), and CME Outfitters. Jordi Rodon reports research funding from 3H Pharmaceuticals; AADI Bioscience, Amgen, AstraZenneca, Beigene, Bicycle Therapeutics, BioAlta, Biotheryx, Blueprint Medicines, Bridgebio Pharma, C4 Therapeutics, Cancer Core Europe, Debio, Fog Pharmaceuticals, ForeBio, FusionPharma, GlaxoSmithKline, Hummingbird, Hutchinson MediPharma, Ideaya, Incyte, Kelun-Biotech, Kinnate, Linnaeus Therapeutics, Loxo Oncology, MapKure, Merck Sharp & Dohme, Merus, Mirati, MonteRosa, Novartis, Nuvectis Pharma, Pfizer, Relay, Roche Pharmaceuticals, Scorpion Therapeutics, Storm Therapeutics, Symphogen, Taiho, Tango Therapeutics, Tyra, Vall d’Hebron Institute of and Oncology/Cancer Core Europe, Yingli; consulting/advisory board role for AADI Bioscience, Amgen, Boxer Capital, Bridgebio, Chinese University of Hong Kong, Cogent Biosciences, EcoR1 Capital, Ellipses Pharma, Guidepoint; IONCTURA, Mekanistic, Merus, Molecular Partners, MonteRosa, Sardona, Tang Advisors, Vall d’Hebron Institute of Oncology/Ministero De Empleo Y Seguridad Social; travel support from European Society for Medical Oncology and Loxo Oncology; and other support from Boxer Capital, Chinese University of Hong Kong, Guidepoint; Tang Advisors, and Vall d’Hebron Institute of Oncology/Ministero De Empleo Y Seguridad Social. Vivek Subbiah reports research funding from Abbvie, Agensys, Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicine, Boston Biomedical, Boston Pharmaceuticals, D3 Bio, Dragonfly Therapeutics, Exelixis, Fujifilm, GlaxoSmith-Kline, Idera Pharmaceuticals, Incyte, Inhibrix, Eli Lilly/Loxo Oncology, MedImmune, NanoCarrier, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, and Vegenics; consulting/advisory role for Abbvie, Astex Pharmaceuticals, AstraZeneca, Bayer, Genmab, Incyte, Lilly/Loxo Oncology, Novartis, Obsidian Therapeutics, Pfizer, Pheon Therapeutics, Regeneron, Relay Therapeutics, Roche, Endeavor Biomedicines, Revolution Medicines; and other support from Helsinn Healthcare, Jazz pharmaceuticals, Incyte, Loxo Oncology/Lilly, Novartis, Relay Therapeutics, Daiichi Sankyo, Illumina, Bayer, Medscape, OncLive, Clinical Care Communications, PERS, and Med learning group. Daniel Karp reports research funding from NIH Grants Clinical Translational Science Award 2019-2024, NCI Core Grant, Clinical Translational 2002–2023, and Myriad Drug Companies; consulting for Black Beret Life Sciences; and advisory board for Affigen and Phosplatin. Michael Curran reports research funding from ImmunoGenesis; and consulting for Agenus, Alligator Bioscience, AstraZeneca, ImmunOS, Hinge Bio, Oncoresponse, Kineta, and Xencor. David S. Hong reports research support from AbbVie, Adaptimmune, Adlai-Nortye, Amgen, Astelles, Astra-Zeneca, Bayer, Biomea, Bristol-Myers Squibb, Daiichi-Sankyo, Deciphera, Eisai, Eli Lilly, Endeavor, Erasca, F. Hoffmann-LaRoche, Fate Therapeutics, Genentech, Genmab, Immunogenesis, Infinity, Kyowa Kirin, Merck, Mirati, Navier, NCI-CTEP, Novartis, Numab, Pfizer, Pyramid Bio, Revolution Medicine, SeaGen, STCube, Takeda, TCR2, Turning Point Therapeutics, VM Oncology; consulting/speaker/advisory roles for 28Bio, Abbvie, Acuta, Adaptimmune, Alkermes, Alpha Insights, Amgen, Affini-T, Astellas, Aumbiosciences, Axiom, Baxter, Bayer, Boxer Capital, BridgeBio, CARSgen, CLCC, COG, COR2ed, Cowen, Ecor1, EDDC, Erasca, Exelixis, Fate Therapeutics, F.Hoffmann-La Roche, Genentech, Gennao Bio, Gilead, GLG, Group H, Guidepoint, HCW Precision Oncology, Immunogenesis, Incyte, Inhibrix, InduPro, Janssen, Jounce Therapeutics Inc, Lan-Bio, Liberium, MedaCorp, Medscape, Novartis, Numab, Oncologia Brasil, ORI Capital, Pfizer, Pharma Intelligence, POET Congress, Prime Oncology, Projects in Knowledge, Quanta, RAIN, Ridgeline, Revolution Medicine SeaGen, Stanford, STCube, Takeda, Tavistock, Trieza Therapeutics, T-Knife, Turning Point Therapeutics, WebMD, YingLing Pharma, and Ziopharm; travel support from AACR, ASCO, CLCC, Bayer, Genmab, SITC, and Telperian; and other ownership interests for Molecular Match (advisor), OncoResponse (founder, advisor), and Telperian (founder, advisor).
Figures


References
-
- Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176:2635–2644. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
