Use of putative hepatoprotective agents as an adjunct to anti-TB treatment in Europe
- PMID: 39959402
- PMCID: PMC11827669
- DOI: 10.5588/ijtldopen.24.0498
Use of putative hepatoprotective agents as an adjunct to anti-TB treatment in Europe
Abstract
Background: Anecdotal information suggests that clinical practice regarding the use of putative hepatoprotective agents in TB treatment varies across countries in the WHO European Region.
Methods: Between November 2023 and May 2024, we conducted a standardised questionnaire survey on the use of putative hepatoprotective agents in patients receiving TB treatment among Tuberculosis Network European Trials Group (TBnet) representatives in countries in the WHO European Region.
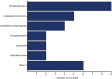
Results: We received valid responses from 37 of 53 countries (69.8%), with 16 (43.2%) reporting regular use of putative hepatoprotective agents during anti-TB treatment. Half of these countries (n = 8) are part of the former Soviet Union. In five countries, these agents are recommended by national guidelines. The most commonly used hepatoprotective agents were silibin/silymarin (n = 9, 56.3%), ursodeoxycholic acid (n = 5, 31.3%), and soy phospholipids (n = 4, 25.0%). Treatment duration varied, with 56.3% (n = 9) using them for less than 1 month, 18.8% (n = 3) for 1-3 months, and 18.8% (n = 3) for 4-6 months.
Conclusions: Putative hepatoprotective agents are widely used as an adjunct to TB treatment in the WHO European Region, particularly in the countries of the former Soviet Union, some of which have included them in their national guidelines.
Keywords: drug-induced liver injury; hepatitis; hepatotoxicity; silibinin; ursodeoxycholic acid.
© 2025 The Authors.
Figures


References
-
- European Centre for Disease Prevention and Control & World Health Organization, Regional Office for Europe . Tuberculosis surveillance and monitoring in Europe 2024: 2022 data. Solna, Sweden: ECDC, 2024.
-
- World Health Organization . WHO consolidated guidelines on tuberculosis. Module 4: Treatment – Drug-susceptible tuberculosis treatment. Geneva, Switzerland: WHO, 2022. - PubMed
-
- World Health Organization . Global tuberculosis report, 2023. Geneva, Switzerland: WHO, 2023.
-
- Huang YS, et al. . Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37(4):924–930. - PubMed
-
- Tostmann A, et al. . Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192–202. - PubMed
LinkOut - more resources
Full Text Sources
