Targeted ErbB4 receptor activation prevents D-galactose-induced neuronal senescence via inhibiting ferroptosis pathway
- PMID: 39959423
- PMCID: PMC11825806
- DOI: 10.3389/fphar.2025.1528604
Targeted ErbB4 receptor activation prevents D-galactose-induced neuronal senescence via inhibiting ferroptosis pathway
Abstract
Background: Neuronal senescence is a common pathological feature of various neurodegenerative diseases, with ferroptosis playing a significant role. This study aims to investigate the role of ErbB4 receptor activation in preventing D-Galactose (D-gal)-induced neuronal senescence.
Methods: Mice subjected to D-gal-induced aging were administered a small molecule ErbB4 receptor agonist (E4A), identified via virtual screening, melatonin, or a combination of both. Behavioral assessments were conducted to evaluate therapeutic efficacy in memory and cognitive functions. Immunofluorescence staining, western blot, and biochemical assays were primarily employed to assess changes in both senescence- and ferroptosis-related molecules in mouse hippocampal tissues in response to each treatment. Additionally, mouse hippocampal HT22 neuronal cell cultures were utilized to corroborate the in vivo findings.
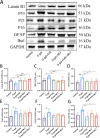
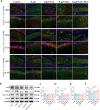
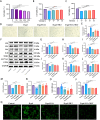
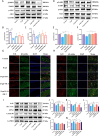
Results: The targeted activation of ErbB4 receptor by E4A significantly ameliorated the behavioral deficits induced by D-gal in mice, demonstrating an effect comparable to that of melatonin, a natural inhibitor of in vivo senescence and ferroptosis. Both E4A and melatonin mitigated D-gal-induced aging in hippocampal neurons of mice. This was evidenced by the upregulation of Lamin B1 and the downregulation of P53, P21, P16, GFAP, and Iba-1 expression levels. Moreover, D-gal treatment markedly decreased the protein expression of the ferroptosis inhibitor Nrf2 while augmenting the expression of the ferroptosis promoter TFRC. These alterations were partially reversed by the individual administration of E4A and melatonin. In vitro studies further corroborated that D-gal treatment significantly and concurrently induced the expression of senescence markers and ferroptosis promoters. However, both E4A and melatonin were able to significantly reverse these changes. Additionally, E4A markedly ameliorated Erastin-induced ferroptosis in mouse hippocampal neuronal cells.
Conlusion: Our findings suggest that targeted activation of ErbB4 receptor may be a viable strategy for treating neuronal senescence by inhibiting ferroptosis, thereby offering a potential therapeutic avenue for senescence-associated neurodegenerative diseases.
Keywords: D-galactose; ErbB4 receptor; ferroptosis; hippocampus; neurodegenerative diseases; neuron; senescence; small molecule.
Copyright © 2025 Dao, Zhang, Liu, Li, Qiao, Cui, Shen, Chen and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bellinger F. P., Bellinger M. T., Seale L. A., Takemoto A. S., Raman A. V., Miki T., et al. (2011). Glutathione peroxidase 4 is associated with neuromelanin in substantia nigra and dystrophic axons in putamen of Parkinson's brain. Mol. Neurodegener. 6 (1), 8. 10.1186/1750-1326-6-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

