Acupuncture mediates the "gut-testis axis" to improve asthenozoospermia
- PMID: 39959619
- PMCID: PMC11827431
- DOI: 10.3389/fendo.2025.1514010
Acupuncture mediates the "gut-testis axis" to improve asthenozoospermia
Abstract
Background: Asthenozoospermia is a common cause of male infertility. Studies have shown that sperm quality and motility are affected by the gut-testis axis that can regulate testicular metabolism and function through the gut microbiota and its metabolites. Acupuncture is an important modality of complementary and alternative medicine. It can improve sperm motility, but it remains unclear whether acupuncture can enhance sperm vitality by influencing the gut-testis axis.
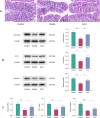
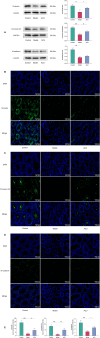
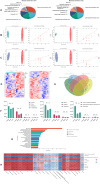
Methods: In this study, sperm quality, testicular pathology, and serum hormone levels were assessed using a cyclophosphamide-induced mouse model. Real-time PCR, a western blot analysis, and immunofluorescence techniques were used to assess the effects of acupuncture on the gut barrier and blood-testis barrier functions. In addition, gut microbiome and metabolomics were used to study the impact of acupuncture on the gut microbiota structure, serum, and testicular metabolites in asthenozoospermic mice. Further validation was obtained by performing a fecal microbiota transplantation (FMT).
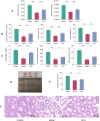
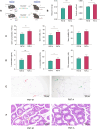
Results: Acupuncture improved the sperm quality; ameliorated testicular pathology; increased serum testosterone (T), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) levels; and repaired gut and blood-testis barrier damage in asthenozoospermic mice. The abundances of Bacteroidota, Firmicutes, Faecalibaculum, and Dubosiella were associated with sperm motility, as shown by a gut microbiome analysis. Serum metabolomics revealed that differentially expressed metabolites (DEMs), such as cytosine and N-oleyl-leucine, were closely related to sperm motility. Testicular metabolomics analysis revealed DEMs, such as 5-fluorouridine and 1-acetylimidazole, were also associated with sperm motility. Furthermore, reproductive function improvements in asthenozoospermic mice through acupuncture were achieved via an FMT.
Conclusion: Acupuncture may alleviate asthenozoospermia symptoms by modulating the gut-testis axis and repairing the gut-testis barrier.
Keywords: acupuncture; asthenozoospermia; gut microbiota; gut-testis axis; metabolomics.
Copyright © 2025 Hao, Xu, Chang, Ren, Wang and Ji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
[Effect of "Zhibian" (BL54)-toward-"Shuidao" (ST28) acupuncture on reproductive function in mice with asthenozoospermia based on mitochondrial apoptosis].Zhongguo Zhen Jiu. 2025 Jan 12;45(1):71-81. doi: 10.13703/j.0255-2930.20231103-k0005. Zhongguo Zhen Jiu. 2025. PMID: 39777882 Chinese.
-
[Mechanism research of "Zhibian" (BL54)-toward-"Shuidao" (ST28) acupuncture technique for improving reproductive function in mice with asthenospermia based on the ferroptosis pathway].Zhongguo Zhen Jiu. 2025 Mar 12;45(3):351-360. doi: 10.13703/j.0255-2930.20240907-k0002. Epub 2025 Jan 3. Zhongguo Zhen Jiu. 2025. PMID: 40097219 Chinese.
-
Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia.Theranostics. 2021 Jan 1;11(6):2822-2844. doi: 10.7150/thno.51869. eCollection 2021. Theranostics. 2021. PMID: 33456575 Free PMC article.
-
[Acupuncture treatment of oligoasthenozoospermia].Zhonghua Nan Ke Xue. 2018 Janurary;24(1):86-90. Zhonghua Nan Ke Xue. 2018. PMID: 30157368 Review. Chinese.
-
Acupuncture for oligospermia and asthenozoospermia: A systematic review and meta-analysis.Medicine (Baltimore). 2021 Dec 3;100(48):e27816. doi: 10.1097/MD.0000000000027816. Medicine (Baltimore). 2021. PMID: 35049183 Free PMC article.
References
-
- World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; (2021), ISBN: ISBN: 9789241547789.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

