Ensitrelvir for the Treatment of Nonhospitalized Adults with COVID-19: Results from the SCORPIO-HR, Phase 3, Randomized, Double-blind, Placebo-Controlled Trial
- PMID: 39960062
- PMCID: PMC12272848
- DOI: 10.1093/cid/ciaf029
Ensitrelvir for the Treatment of Nonhospitalized Adults with COVID-19: Results from the SCORPIO-HR, Phase 3, Randomized, Double-blind, Placebo-Controlled Trial
Abstract
Background: Ensitrelvir, a severe acute respiratory syndrome coronavirus-2 main protease inhibitor, has demonstrated clinical and virologic efficacy in previous studies.
Methods: In this global phase 3 trial, nonhospitalized adults with mild-to-moderate coronavirus disease 2019 (COVID-19) and symptom onset within 5 days were randomized (1:1) to receive once-daily ensitrelvir (375 mg day 1, 125 mg days 2-5) or blinded matching placebo. The primary endpoint was the restricted mean time to sustained (≥2 days) resolution of 15 COVID-19 symptoms, recorded in participant daily diaries, through day 29 in participants starting treatment within 3 days after symptom onset. Virologic efficacy and safety were assessed.
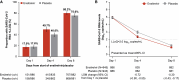
Results: Of 2093 participants, 1888 started treatment within 3 days after symptom onset. Mean time to symptom resolution was 12.5 and 13.1 days with ensitrelvir and placebo, respectively (difference, -0.6 days; 95% confidence interval, -1.38 to 0.19; P = .14). On day 4, ensitrelvir reduced least-squares mean RNA by 0.72 log10 copies/mL more than placebo (95% confidence interval, 0.55-0.90). Among those with positive viral cultures at enrollment, 274/287 (95.5%) ensitrelvir-treated versus 210/280 (75.0%) placebo-treated participants had negative cultures on day 4. RNA rebound was similar (<1.5%) between groups. The proportion of participants with ≥1 adverse event was similar with ensitrelvir (61.5%) and placebo (60.6%). No treatment-related serious adverse events or deaths occurred. Three (0.3%) ensitrelvir-treated and 1 (0.1%) placebo-treated participants had COVID-19-related hospitalizations by day 29.
Conclusions: Despite the evidence of antiviral activity with ensitrelvir, this trial did not demonstrate a significant difference in time to sustained symptom resolution.
Clinical trials registration number: NCT05305547.
Keywords: COVID-19; ensitrelvir; high risk; symptom resolution; viral rebound.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures

Comment in
-
Antiviral Trials-Navigating the Shifting Sands of a Pandemic.Clin Infect Dis. 2025 Jul 18;80(6):1245-1246. doi: 10.1093/cid/ciaf031. Clin Infect Dis. 2025. PMID: 39960845 Free PMC article. No abstract available.
References
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Last updated 26 March 2024. Accessed 28 August 2024.
-
- World Health Organization . TAG-VE statement on Omicron sublineages BQ.1 and XBB. Available at: https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sub.... Accessed 28 August 2024.
-
- Lazarus JV, Wyka K, White TM, et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med 2023; 29:366–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1AI068636/National Institute of Allergy and Infectious Diseases of the National Institutes of Health
- UM1AI106701/National Institute of Allergy and Infectious Diseases of the National Institutes of Health
- UM1AI068634/National Institute of Allergy and Infectious Diseases of the National Institutes of Health
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

