"Naked Nickel"-Catalyzed Heteroaryl-Heteroaryl Suzuki-Miyaura Coupling
- PMID: 39960149
- PMCID: PMC12105700
- DOI: 10.1002/anie.202424051
"Naked Nickel"-Catalyzed Heteroaryl-Heteroaryl Suzuki-Miyaura Coupling
Abstract
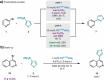
In this article, we report that the air-stable "naked nickel", [Ni(4-CF3stb)3], is a competent catalyst in the catalytic Suzuki-Miyaura cross-coupling reaction (SMC) between heteroaryl bromides and heteroaromatic boron-based nucleophiles. The catalytic system is characterized by its ability to avoid decomposition or deactivation in the presence of multiple Lewis basic sites. The protocol permits the formation of C‒C bonds between two heteroaryl moieties in the absence of complex exogenous ligands, thus minimizing screening procedures and simplifying reaction setups. This method accommodates combinations of distinct 6-membered heteroaryl bromides and 5- and 6-membered heterocyclic B-based nucleophiles.
Keywords: Boron; Cross‐coupling; Heterocycles; Nickel; Synthetic methods.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brown D. G., Boström J., J. Med. Chem. 2016, 59, 4443–4458. - PubMed
-
- Roughley S. D., Jordan A. M., J. Med. Chem. 2011, 54, 3451–3479. - PubMed
-
- Marshall C. M., Federice J. G., Bell C. N., Cox P. B., Njardarson J. T., J. Med. Chem. 2024, 67, 11622–11655. - PubMed
-
- West M. J., Watson A. J. B., Org. Biomol. Chem. 2019, 17, 5055–5059. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

