Decellularized dermis allograft for the treatment of venous leg ulceration: the DAVE RCT
- PMID: 39960153
- PMCID: PMC11831364
- DOI: 10.1093/bjs/znae330
Decellularized dermis allograft for the treatment of venous leg ulceration: the DAVE RCT
Abstract
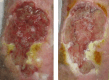
Background: Venous leg ulcers (VLUs) cause significant impairment to patients' quality of life (QoL) and up to 30% do not heal at 6 months. Decellularized dermis (DCD) allografts have been shown to be effective in improving healing rates of diabetic foot ulcers in RCTs. The DAVE RCT aimed to determine whether DCD is an effective, safe, and cost-effective treatment adjunct for VLUs.
Methods: This was a multicentre RCT performed in the UK. Patients with lower limb ulcers ≥18 years with VLU, venous incompetence on duplex ultrasound, an ankle : brachial pressure index ≥ 0.8 and an index VLU present for at least 3 months and ≥2 cm2 in size were included. Patients were randomized to either the intervention (DCD graft and standard of care) or control arm (standard of care alone). The primary outcome was the proportion of patients with a healed index ulcer at 12 weeks.
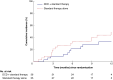
Results: From October 2019 to October 2022, 36 and 35 participants were randomized into the intervention and control arms respectively. Patient characteristics at baseline were similar between groups. Healing was recorded in 5.7% of the intervention group and 15.2% in the control group (OR 0.34, 95% c.i. 0.03 to 2.31). There were no significant differences in the secondary outcomes including the percentage change in ulcer area, time to healing, recurrence rates, and QoL. There were five serious adverse events attributed to DCD application. Early trial termination was advised after the interim data analysis due to a lower-than-expected primary outcome rate (11.3%).
Conclusions: Decellularized dermis grafts did not improve healing rates of venous leg ulcers, although the trial was terminated early due to poor healing rates in both the intervention and control arms. Important clinical benefits or harms of decellularized dermis grafts could not be excluded due to the small sample size.
Trial registration: ISRCTN 21541209.
© The Author(s) 2025. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures
References
-
- Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol 2005;15:175–184 - PubMed
-
- Carradice D, Mazari FAK, Samuel N, Allgar V, Hatfield J, Chetter IC. Modelling the effect of venous disease on quality of life. Br J Surg 2011;98:1089–1098 - PubMed
-
- Lal BK. Venous ulcers of the lower extremity: definition, epidemiology, and economic and social burdens. Semin Vasc Surg 2015;28:3–5 - PubMed
-
- Gohel MS, Taylor M, Earnshaw JJ, Heather BP, Poskitt KR, Whyman MR. Risk factors for delayed healing and recurrence of chronic venous leg ulcers—an analysis of 1324 legs. Eur J Vasc Endovasc Surg 2005;29:74–77 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources