Target Agnostic Photoaffinity Labelling by Sulfonylhydrazones
- PMID: 39960219
- PMCID: PMC12015381
- DOI: 10.1002/anie.202408701
Target Agnostic Photoaffinity Labelling by Sulfonylhydrazones
Abstract
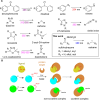
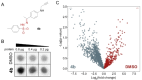
Photoaffinity labeling is a widely used methodology for interrogating small molecule-protein interactions. However, these applications are limited by the few photo-crosslinkers that typically modify the affinity and the binding mode of the original ligand. Here, we report the development of new target agnostic photoaffinity warheads, sulfohydrazones that form a reactive carbene upon UV irradiation. Careful optimization of the reaction conditions allowed us to effectively label five different amino acid residues in proteins. Our approach turned biologically relevant hydrazones and sulfohydrazones to intrinsically irreversible covalent binders without structural modifications by photoactivation as demonstrated on monoamine oxidase A (MAO-A) enzyme and STAT5b (Signal transducer and activator of transcription 5b) transcription factor. Sulfohydrazones are readily accessible by transforming the corresponding carbonyl group of a ligand or a suitable tag that extends the application domain of the method for any ligands exemplified by conditional labelling of the acetylcholine esterase enzyme and the oncogenic mutant of GTP-ase KRasG12D.
Keywords: KRas; STAT; acetylcholine esterase; photoaffinity; sulfohydrazone.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Experimental and Computational Evaluation of Piperonylic Acid Derived Hydrazones Bearing Isatin Moieties as Dual Inhibitors of Cholinesterases and Monoamine Oxidases.ChemMedChem. 2019 Jul 17;14(14):1359-1376. doi: 10.1002/cmdc.201900277. Epub 2019 Jul 8. ChemMedChem. 2019. PMID: 31177620
-
Photoaffinity Labeling of Pentameric Ligand-Gated Ion Channels: A Proteomic Approach to Identify Allosteric Modulator Binding Sites.Methods Mol Biol. 2017;1598:157-197. doi: 10.1007/978-1-4939-6952-4_7. Methods Mol Biol. 2017. PMID: 28508361
-
Oxadiazolines as Photoreleasable Labels for Drug Target Identification.J Am Chem Soc. 2024 Oct 2;146(39):26759-26765. doi: 10.1021/jacs.4c06936. Epub 2024 Sep 17. J Am Chem Soc. 2024. PMID: 39288302
-
Photoaffinity labeling and its application in structural biology.Biochemistry (Mosc). 2007 Jan;72(1):1-20. doi: 10.1134/s0006297907010014. Biochemistry (Mosc). 2007. PMID: 17309432 Review.
-
Photoaffinity labelling strategies for mapping the small molecule-protein interactome.Org Biomol Chem. 2021 Sep 22;19(36):7792-7809. doi: 10.1039/d1ob01353j. Org Biomol Chem. 2021. PMID: 34549230 Free PMC article. Review.
Cited by
-
Re-participation intention in individuals playing tennis for recreational purposes: investigation of differences based on low and high involvement.Front Psychol. 2025 Mar 10;16:1546405. doi: 10.3389/fpsyg.2025.1546405. eCollection 2025. Front Psychol. 2025. PMID: 40129495 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- RRF-2.3.1-21-2022-00015/National Research, Development and Innovation Office
- K135150/National Research, Development and Innovation Office
- 2020-1.1.6-JÖVŐ-2021-00004/National Research, Development and Innovation Office
- NAP 3.0/Magyar Tudományos Akadémia
- János Bolyai Research Fellowship/Magyar Tudományos Akadémia
LinkOut - more resources
Full Text Sources
Miscellaneous

