Structure and function of MDM2 and MDM4 in health and disease
- PMID: 39960347
- PMCID: PMC12096895
- DOI: 10.1042/BCJ20240757
Structure and function of MDM2 and MDM4 in health and disease
Abstract
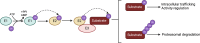
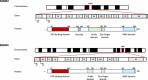
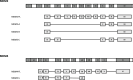
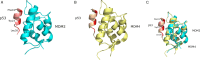
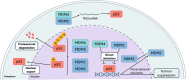
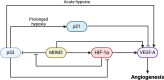
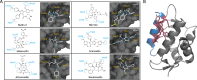
Both mouse double-minute 2 (MDM2), an E3 ubiquitin ligase, and its closely related paralog, MDM4, which lacks E3 activity, play central roles in cellular homeostasis. MDM-linked dysfunction is associated with an increased risk of oncogenesis, primarily through targeting the tumor suppressor protein p53 for ubiquitination and degradation. Recent studies have revealed multifaceted roles of MDM proteins that are p53 independent with implications for their oncogenic properties. This review aims to provide an overview of MDM2 and MDM4, by assessing gene and protein structure and implications for protein-protein interactions and functions in cell and animal physiology. We also explore MDM2 and MDM4 role(s) in angiogenesis, a critical feature of solid tumor growth and progression. Finally, we discuss the current landscape in the development of MDM2 and MDM4 inhibitors for cancer therapy.
Keywords: 26S proteasome; E3 ubiquitin ligase; MDM2; MDM4; cancer; p53.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

