IL-15 transpresentation by ovarian cancer cells improves CD34+ progenitor-derived NK cell's anti-tumor functionality
- PMID: 39960378
- PMCID: PMC11834524
- DOI: 10.1080/2162402X.2025.2465010
IL-15 transpresentation by ovarian cancer cells improves CD34+ progenitor-derived NK cell's anti-tumor functionality
Abstract
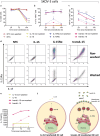
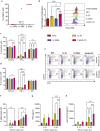
Ovarian cancer (OC) is the most lethal gynecological malignancy. As high numbers of Natural Killer (NK) cells in ascites associate with improved survival, the adoptive transfer of allogeneic NK cells is an attractive therapeutic strategy. An approach to further improve NK cell expansion and anti-tumor functionality post-infusion includes IL-15 transpresentation (transIL-15), which involves surface expression of the IL-15 cytokine bound to IL-15Rα. However, others have substantiated that systemic administration of ALT/N-803, a soluble molecule mimicking transIL-15, leads to T cell-mediated rejection of the infused allogeneic NK cell product. In addition, whether transIL-15 induce superior expansion and functionality of our hematopoietic progenitor cell-derived NK cells (HPC-NK) remains understudied. Here, we propose to transfect OC cells with IL-15 and IL-15Rα mRNA and evaluate HPC-NK cell stimulation in vitro. Co-transfection of both mRNAs resulted in surface co-expression of both components, thus mimicking the transIL-15. Importantly, co-culture of HPC-NK cells with transIL-15 OC cells resulted in superior proliferation, IFNγ production, cytotoxicity and granzyme B secretion. Furthermore, we observed uptake of IL-15Rα by HPC-NK cells when co-cultured with transIL-15 OC cells, which associates with NK cell long-term proliferation and survival. Superior killing and granzyme B secretion were also observed in transIL-15 OC spheroids. Our results demonstrate that local delivery of IL-15 and IL-15Rα mRNA to OC tumors may be a safer strategy to boost HPC-NK cell therapy of OC through IL-15 transpresentation.
Keywords: IL-15 transpresentation; NK cell immunotherapy; mRNA delivery; ovarian cancer.
Conflict of interest statement
Roland Brock is co-founder and co-owner of Mercurna B. V. and RIBOPRO B. V., companies that develop mRNA-based therapeutics.
Figures


References
-
- Hoogstad-van Evert JS, Maas RJ, van der Meer J, Cany J, van der Steen S, Jansen JH, Miller JS, Bekkers R, Hobo W, Massuger L, et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget. 2018;9(78):34810–34820. doi:10.18632/oncotarget.26199. - DOI - PMC - PubMed
-
- de Jonge P, van Hauten PMM, Janssen LD, de Goede AL, Berrien-Elliott MM, van der Meer JMR, Mousset CM, Roeven MWH, Foster M, Blijlevens N, et al. Good manufacturing practice production of CD34(+) progenitor-derived NK cells for adoptive immunotherapy in acute myeloid leukemia. Cancer Immunol Immunother. 2023;72(10):3323–3335. doi:10.1007/s00262-023-03492-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
