Zanubrutinib in Japanese treatment-naive and relapsed/refractory patients with Waldenström macroglobulinemia and CLL/SLL
- PMID: 39960615
- PMCID: PMC11922998
- DOI: 10.1007/s12185-025-03925-1
Zanubrutinib in Japanese treatment-naive and relapsed/refractory patients with Waldenström macroglobulinemia and CLL/SLL
Abstract
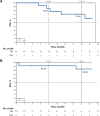
Zanubrutinib is a selective second-generation Bruton tyrosine kinase inhibitor approved in various B-cell malignancies globally. The phase 1/2 BGB-3111-111 study evaluated the efficacy and safety of zanubrutinib 160 mg twice daily orally in Japanese patients with treatment-naive or relapsed/refractory mature B-cell malignancies. Here, efficacy results from Part 2 in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL; n = 19) and Waldenström macroglobulinemia (WM; n = 19), and safety results from Parts 1 (N = 6) and 2 (N = 49) are presented, with the first dose between 30 January, 2020, and 31 October, 2022. As of 10 May, 2023, investigator-assessed overall response rates were 100% (19/19) and 94.7% (18/19) in CLL/SLL and WM, respectively, with median follow-up of 27.9 and 26.8 months; 24-month progression-free survival rates were 71.4% and 100% in treatment-naive and relapsed/refractory CLL/SLL and 83.9% and 100% in treatment-naive and relapsed/refractory WM, respectively. In patients with B-cell malignancies, any-grade treatment-emergent adverse events (TEAEs) occurred in 53 (96.4%) and serious TEAEs in 18 (32.7%). Common TEAEs were platelet count decreased (18.2%), pyrexia (18.2%), COVID-19 (14.5%), and neutrophil count decreased (12.7%). With median follow-up > 2 years, zanubrutinib demonstrated durable efficacy in Japanese patients with CLL/SLL or WM and a favorable safety profile consistent with global phase 3 studies.
Keywords: Bruton tyrosine kinase; Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma; Japanese; Waldenström Macroglobulinemia; Zanubrutinib.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Takayuki Ishikawa, Kazuyuki Shimada, Kohmei Kubo, Takeshi Kondo, Tomoaki Fujisaki, Shingo Kurahashi, Rika Sakai, Tatsuro Jo, Tomonori Nakazato, Masahiro Takeuchi: No conflict of interest to disclose. Koji Izutsu: Honoraria from Ono Pharmaceutical, Janssen; research funding from AstraZeneca, AbbVie, Incyte, Bristol Myers Squibb, Novartis, Janssen, Yakult, Daiichi Sankyo, Chugai, BeiGene, Genmab. Katsuya Fujimoto: Research funding from Parexel International Co, Ltd, Insight Biosciences Japan, LLC. Kazutaka Sunami: Honoraria from Celgene, Bristol Myers Squibb, Takeda Pharmaceutical, Sanofi; research funding from Ono Pharmaceutical, MSD, Celgene, AbbVie G.K., Takeda Pharmaceutical, Sanofi, Bristol Myers Squibb, Daiichi Sankyo, Alexion Pharma, GSK, Otsuka Pharmaceutical, Novartis Pharma, Astellas, Amgen, Janssen Pharma, Chugai Pharmaceutical, Kyowa Kirin, Pfizer. Senji Kasahara: Honoraria from Daiichi Sankyo; research funding from Novartis Pharma, Astellas Pharma, Daiichi Sankyo. Aileen Cohen: Consultant and equity holder with BeiGene. Motohisa Takai, Jinhua Zhong: Employment and stock options from BeiGene. Koji Nagafuji: An editor of International Journal of Hematology during the review process.
Figures

References
-
- Tomlinson R. Chronic lymphocytic leukaemia: an updated approach to diagnosis and management in general practice. Aust Fam Physician. 2017;46(7):493–6. - PubMed
-
- Kim H-O. Development of BTK inhibitors for the treatment of B-cell malignancies. Arch Pharmacal Res. 2019;42(2):171–81. - PubMed
-
- Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–32. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

