Microbial ecology of serpentinite-hosted ecosystems
- PMID: 39961017
- PMCID: PMC11931622
- DOI: 10.1093/ismejo/wraf029
Microbial ecology of serpentinite-hosted ecosystems
Abstract
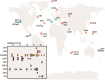
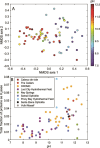
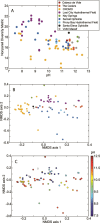
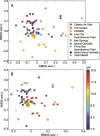
Serpentinization, the collective set of geochemical reactions initiated by the hydration of ultramafic rock, has occurred throughout Earth history and is inferred to occur on several planets and moons in our solar system. These reactions generate highly reducing conditions that can drive organic synthesis reactions potentially conducive to the emergence of life, while concomitantly generating fluids that challenge life owing to hyperalkalinity and limited inorganic carbon (and oxidant) availability. Consequently, the serpentinite-hosted biosphere offers insights into the earliest life, the habitable limits for life, and the potential for life on other planets. However, the support of abundant microbial communities by serpentinites was only recognized ~20 years ago with the discovery of deep-sea hydrothermal vents emanating serpentinized fluids. Here, we review the microbial ecology of both marine and continental serpentinization-influenced ecosystems in conjunction with a comparison of publicly available metagenomic sequence data from these communities to provide a global perspective of serpentinite microbial ecology. Synthesis of observations across global systems reveal consistent themes in the diversity, ecology, and functioning of communities. Nevertheless, individual systems exhibit nuances due to local geology, hydrology, and input of oxidized, near-surface/seawater fluids. Further, several new (and old) questions remain including the provenance of carbon to support biomass synthesis, the physical and chemical limits of life in serpentinites, the mode and tempo of in situ evolution, and the extent that modern serpentinites serve as analogs for those on early Earth. These topics are explored from a microbial perspective to outline key knowledge-gaps for future research.
Keywords: Serpentinite; acetogens; astrobiology; carbon fixation; early earth; hydrogen; hydrogenase; methanogens; microbial ecology.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare a lack of conflicts of interest in the preparation of this manuscript.
Figures



References
-
- McCollom TM, Bach W. Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks. Geochim Cosmochim Acta 2009;73:856–75. 10.1016/j.gca.2008.10.032 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

