Mitochondrial Transplantation via Magnetically Responsive Artificial Cells Promotes Intracerebral Hemorrhage Recovery by Supporting Microglia Immunological Homeostasis
- PMID: 39961067
- PMCID: PMC11962678
- DOI: 10.1002/adma.202500303
Mitochondrial Transplantation via Magnetically Responsive Artificial Cells Promotes Intracerebral Hemorrhage Recovery by Supporting Microglia Immunological Homeostasis
Abstract
The immune-inflammatory responses in the brain represent a key therapeutic target to ameliorate brain injury following intracerebral hemorrhage (ICH), where pro-inflammatory microglia and its mitochondrial dysfunction plays a pivotal role. Mitochondrial transplantation is a promising strategy to improve the cellular mitochondrial function and thus modulate their immune properties. However, the transplantation of naked mitochondria into the brain has been constrained by the peripheral clearance and the difficulty in achieving selective access to the brain. Here, a novel strategy for mitochondrial transplantation via intravenous injection of magnetically responsive artificial cells (ACs) are proposed. ACs can protect the loaded mitochondria and selectively accumulate around the lesion under an external magnetic field (EMF). In this study, mitochondria released from ACs can effectively improve microglial mitochondrial function, attenuate their pro-inflammatory attributes, and elevate the proportion of immunosuppressive microglia. In this way, microglia immune homeostasis in the brain is reestablished, and inflammation is attenuated, ultimately promoting functional recovery. This study presents an effective approach to transplant mitochondria into the brain, offering a promising alternative to modulate the immune-inflammatory cascade in the brain following ICH.
Keywords: artificial cells; immune homeostasis; intracerebral hemorrhage; magnetic drug targeting; mitochondrial transplantation.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

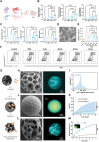





References
-
- Li Q., Yakhkind A., Alexandrov A. W., Alexandrov A. V., Anderson C. S., Dowlatshahi D., Frontera J. A., Hemphill J. C., Ganti L., Kellner C., May C., Morotti A., Parry‐Jones A., Sheth K. N., Steiner T., Ziai W., Goldstein J. N., Mayer S. A., Stroke 2024, 55, 494. - PubMed
-
- Cordonnier C., Demchuk A., Ziai W., Anderson C. S., Lancet 2018, 392, 1257. - PubMed
-
- Shi K., Tian D.‐C., Li Z.‐G., Ducruet A. F., Lawton M. T., Shi F.‐D., Lancet Neurol. 2019, 18, 1058. - PubMed
-
- Xue M., Yong V. W., Lancet Neurol. 2020, 19, 1023. - PubMed
-
- Ungprasert P., Matteson E. L., Thongprayoon C., Stroke 2016, 47, 356. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

