Profiling of the macrophage response to polypropylene mesh burden in vivo
- PMID: 39961254
- PMCID: PMC12225754
- DOI: 10.1016/j.biomaterials.2025.123177
Profiling of the macrophage response to polypropylene mesh burden in vivo
Abstract
Pelvic organ prolapse (POP) surgical repair with polypropylene mesh (PPM) offers improved anatomical outcomes compared to reconstruction using native tissue. However, PPM repair is hampered by complications, most commonly pain or mesh exposure, occurring in over 10 % of cases. This maladaptive response is, in part, attributed to the host response to a foreign material. Previous studies have demonstrated that mesh properties, such as weight, pore size, and porosity, influence downstream outcomes. In addition, computational models and in vivo mechanistic studies demonstrate that mesh deforms after tensioning in prolapse surgery resulting in collapsed pores and wrinkles. To further investigate the role of pore collapse in mesh complications, PPM was implanted flat, or in configurations that would deform upon tensioning in a POP repair surgery using a non-human primate model. After twelve weeks, we analyzed mesh-tissue complexes to characterize the overall host response, profile the macrophage response, and observe the influence of macrophages in downstream healing outcomes that may lead to complications. The results confirm that mesh deformations reproduce mesh exposure and thinning of vagina. In the PPM configurations with the greatest deformation, mesh burden was the highest, which resulted in an overall decrease in the number of cells within the implantation site. Among the cells that were present, we observed a predominance of M1 pro-inflammatory macrophages. While flat mesh was associated with an organized cellular response, deformed mesh led to an increasingly disorganized response as mesh burden increased. Nearly half of the responding macrophages expressed markers associated both with M1 and M2 phenotypes concurrently, suggesting the possibility of newly recruited macrophages responding even 12 weeks after implantation and/or a repetitive microinjury in which macrophages are continuously recruited and polarized without resolution of the host response. Biochemically, we observed a predominantly M1 pro-inflammatory signaling environment and decreased collagen content as a response to implanted mesh. This study evidences the importance of PPM mesh properties, which may alter mesh burden upon tensioning and impact downstream healing outcomes and emphasizes the need for devices that maintain their geometry following implantation in POP surgical repair.
Keywords: Host response; Macrophage; Mesh burden; Pelvic organ prolapse; Polypropylene mesh; Stress shielding.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Pamela Moalli reports financial support was provided by National Institutes of Health. Bryan Brown reports financial support was provided by National Institutes of Health. Pamela Moalli reports equipment, drugs, or supplies was provided by Coloplast Corp. Marrisa Therriault reports financial support was provided by National Center for Advancing Translational Sciences. Pamela Moalli reports a relationship with Hologic Inc that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

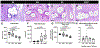
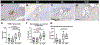



References
-
- DeLancey JO, The anatomy of the pelvic floor. Curr Opin Obstet Gynecol, 6(4): p. 313–6 (1994) - PubMed
-
- Wood LN, Anger JT, Urinary incontinence in women. BMJ 349, g4531 (2014). - PubMed
-
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, & Clark AL, Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89, 501–506 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

