ArfGAP3 Protects Mitochondrial Function and Promotes Autophagy Through Rab5a-Mediated Signals in Ageing Skeletal Muscle
- PMID: 39961359
- PMCID: PMC11832210
- DOI: 10.1002/jcsm.13725
ArfGAP3 Protects Mitochondrial Function and Promotes Autophagy Through Rab5a-Mediated Signals in Ageing Skeletal Muscle
Abstract
Background: Few researches have investigated the molecular mechanism responsible for the age-related loss of the pelvic floor muscle (PFM) mass and functionality-a pivotal contributor to pelvic organ prolapse and diminished physical well-being. ADP ribosylation factor GTPase activating protein 3 (ArfGAP3) is a member of ArfGAPs, which regulates the vesicular trafficking pathway and intracellular proteins transporting. However, its effects on skeletal muscle ageing remain largely unknown.
Methods: Mouse models of natural ageing and D-gal (D-galactose)-induced ageing were subject to analyse the structure, function and pathological alterations of the PFM and the expression of ArfGAP3. Stable ArfGAP3 knockdown and overexpression C2C12 cell lines were established to investigate the anti-senescence effects of ArfGAP3 and the underlying mechanisms in ageing process, complemented by Rab5a genetic intervention and mRFP-GFP-LC3 adenoviral particles transfection. In vivo experiments entailed ArfGAP3 overexpression in mice alongside autophagy inhibitor treatment, with assessments encompassing tissue mass, bladder leak point pressure (BLPP), submicroscopic structure, antioxidative stress system and muscle regeneration.
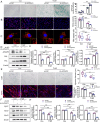
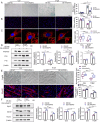
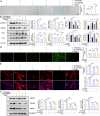
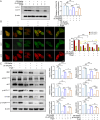
Results: Aged (24-month-old) mice exhibited significant physiological alterations in PFMs, including decreased muscle mass, diminished cross-sectional area (CSA), deteriorated supporting function (as evidenced by reduced BLPP), impaired autophagy and increased levels of oxidative stress (p < 0.001). Utilizing ageing C2C12 model, we observed a dose-dependent relationship between D-gal induction and cellular senescence, impaired differentiation and mitochondrial damage. Remarkably, the expression levels of ArfGAP3 were markedly downregulated in both in vitro and in vivo ageing models. Knockdown of ArfGAP3 exacerbated impaired differentiation potential and induced aberrant mitochondrial morphology and functional dysfunction in ageing C2C12 myoblasts, whereas ArfGAP3 overexpression largely mitigated these effects. Mechanistically, our findings revealed an interplay between ArfGAP3 and Rab5a, indicating their coordinated regulation. ArfGAP3-mediated activation of Rab5a-associated autophagy and IRS1-AKT-mTOR signalling pathways during cellular senescence and myogenesis was identified, leading to enhanced autophagic flux and improved resistance to oxidative stress. In vivo, ArfGAP3 overexpression ameliorated D-gal-induced loss of muscle mass and function, while promoting antioxidant responses and muscle regeneration in mice. However, these protective effects of ArfGAP3 overexpression were extinguished by autophagy inhibition.
Conclusions: Our study uncovers the significant role of ArfGAP3 in enhancing differentiation capacity and mitochondrial function through mediating Rab5a expression to activate IRS1-AKT-mTOR signalling pathways and promote autophagy during the ageing process. These findings underscore the potential of ArfGAP3 as a promising therapeutic target for ameliorating the decline in skeletal muscle function associated with ageing.
Keywords: ArfGAP3; Rab5a; ageing; autophagy; pelvic floor muscle.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Partridge L., Deelen J., and Slagboom P. E., “Facing Up to the Global Challenges of Ageing,” Nature 561 (2018): 45–56. - PubMed
-
- Zeleke B. M., Bell R. J., Billah B., and Davis S. R., “Symptomatic Pelvic Floor Disorders in Community‐Dwelling Older Australian Women,” Maturitas 85 (2016): 34–41. - PubMed
MeSH terms
Substances
Grants and funding
- 82371639/National Natural Science Foundation of China
- 82301828/National Natural Science Foundation of China
- 81971364/National Natural Science Foundation of China
- 2021YFC2701300/National Key Research and Development Program of China
- 2021YFC2701302/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

