Predictors of early disability accumulation in newly diagnosed multiple sclerosis: clinical, imaging and cerebrospinal fluid measures
- PMID: 39961655
- PMCID: PMC12418557
- DOI: 10.1136/jnnp-2024-335037
Predictors of early disability accumulation in newly diagnosed multiple sclerosis: clinical, imaging and cerebrospinal fluid measures
Abstract
Background: A growing arsenal of treatment options for relapsing multiple sclerosis (RMS) emphasises the need for early prognostic biomarkers. While evidence for individual markers exists, comprehensive analyses at the time of diagnosis are sparse.
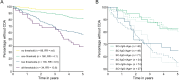
Methods: Brain and spinal cord lesion numbers, cerebrospinal fluid parameters, initial symptoms, and Expanded Disability Status Scale (EDSS) score were determined at the time of diagnosis. Confirmed disability accumulation (CDA), defined as a sustained EDSS increase over 6 months, was determined during a 5-year follow-up. All-subsets multivariable logistic regression was performed to identify predictors of CDA. Model performance was assessed via receiver operating characteristic analysis, and individual risks were calculated. Analyses were repeated with progression independent of relapse activity (PIRA) as an outcome.
Results: 113/417 (27.1%) people with RMS experienced CDA on follow-up. Intrathecal IgG synthesis, a higher number of spinal cord lesions, age and polysymptomatic manifestation were identified as independent predictors of CDA. The resulting prediction model yielded an area under the curve (AUC) of 0.75 with a 95% CI of 0.70 to 0.80. Individuals exceeding the optimal thresholds for the three most significant predictors had a 61.8% likelihood of experiencing CDA, whereas those below all three thresholds had a CDA rate of 4.5%. The only significant baseline predictor differentiating PIRA from relapse-associated worsening was a higher number of spinal cord lesions (AUC=0.64, 95% CI 0.54 to 0.74).
Conclusions: Intrathecal IgG synthesis, spinal cord lesion number, age and polysymptomatic manifestation are independent predictors of early CDA in newly diagnosed RMS.
Keywords: CLINICAL NEUROLOGY; CSF; MRI; MULTIPLE SCLEROSIS; NEUROIMMUNOLOGY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None of the authors have reported conflicts of interest directly related to this study. KG has received reimbursement for traveling expenses from UCB and Viatris. AB has received consulting and/or speaker fees from Alexion, Biogen, Celgene, Horizon, Novartis, Roche and Sandoz/Hexal. His institution has received compensation for clinical trials from Alexion, Biogen, Merck, Novartis, Roche and Sanofi Genzyme. JSK has received speaker fees for Novartis. He is a shareholder of Bonescreen. BH has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon and TG therapeutics; his institution received research grants from Roche for multiple sclerosis research. He has received honoraria for counselling (Gerson Lehrmann Group). He holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralising antibodies to interferon.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
