Local weakening of cell-extracellular matrix adhesion triggers basal epithelial tissue folding
- PMID: 39962267
- PMCID: PMC11961693
- DOI: 10.1038/s44318-025-00384-6
Local weakening of cell-extracellular matrix adhesion triggers basal epithelial tissue folding
Abstract
During development, epithelial sheets sculpt organs by folding, either apically or basally, into complex 3D structures. Given the presence of actomyosin networks and cell adhesion sites on both sides of cells, a common machinery mediating apical and basal epithelial tissue folding has been proposed. However, unlike for apical folding, little is known about the mechanisms that regulate epithelial folding towards the basal side. Here, using the Drosophila wing imaginal disc and combining genetic perturbations and computational modeling, we demonstrate opposing roles for cell-cell and cell-extracellular matrix (ECM) adhesion systems during epithelial folding. While cadherin-mediated adhesion, linked to actomyosin network, regulates apical folding, a localized reduction on integrin-dependent adhesion, followed by changes in cell shape and reorganization of the basal actomyosin cytoskeleton and E-Cadherin (E-Cad) levels, is necessary and sufficient to trigger basal folding. These results suggest that modulation of the cell mechanical landscape through the crosstalk between integrins and cadherins is essential for correct epithelial folding.
Keywords: Actomyosin; Cadherins; Constricting Forces; Integrins.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures







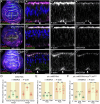
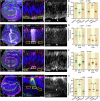
Similar articles
-
Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms.Nat Commun. 2018 Nov 5;9(1):4620. doi: 10.1038/s41467-018-06497-3. Nat Commun. 2018. PMID: 30397306 Free PMC article.
-
Epithelial organ shape is generated by patterned actomyosin contractility and maintained by the extracellular matrix.PLoS Comput Biol. 2020 Aug 20;16(8):e1008105. doi: 10.1371/journal.pcbi.1008105. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32817654 Free PMC article.
-
Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks.PLoS Genet. 2020 Jun 1;16(6):e1008717. doi: 10.1371/journal.pgen.1008717. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32479493 Free PMC article.
-
Patterns in space: coordinating adhesion and actomyosin contractility at E-cadherin junctions.Cell Commun Adhes. 2013 Dec;20(6):201-12. doi: 10.3109/15419061.2013.856889. Epub 2013 Nov 8. Cell Commun Adhes. 2013. PMID: 24205985 Review.
-
Platelets with wings: the maturation of Drosophila integrin biology.Curr Opin Cell Biol. 2003 Oct;15(5):607-13. doi: 10.1016/s0955-0674(03)00102-9. Curr Opin Cell Biol. 2003. PMID: 14519396 Review.
References
-
- Brown NH (1993) Integrins hold Drosophila together. BioEssays 15:383–390 - PubMed
-
- Chalut KJ, Paluch EK (2016) The actin cortex: a bridge between cell shape and function. Dev Cell 38:571–573 - PubMed
-
- Christodoulou N, Skourides PA (2015) Cell-autonomous Ca(2+) flashes elicit pulsed contractions of an apical actin network to drive apical constriction during neural tube closure. Cell Rep 13:2189–2202 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

