Compartmentalized dendritic plasticity in the mouse retrosplenial cortex links contextual memories formed close in time
- PMID: 39962274
- PMCID: PMC11893454
- DOI: 10.1038/s41593-025-01876-8
Compartmentalized dendritic plasticity in the mouse retrosplenial cortex links contextual memories formed close in time
Abstract
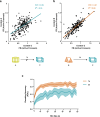
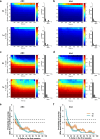
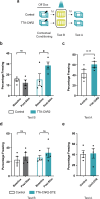
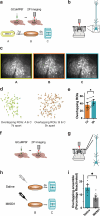
Events occurring close in time are often linked in memory, and recent studies suggest that such memories are encoded by overlapping neuronal ensembles. However, the role of dendritic plasticity mechanisms in linking memories is unknown. Here we show that memory linking is dependent not only on neuronal ensemble overlap in the mouse retrosplenial cortex, but also on branch-specific dendritic allocation mechanisms. The same dendritic segments are preferentially activated by two linked (but not independent) contextual memories, and spine clusters added after each of two linked (but not independent) contextual memories are allocated to the same dendritic segments. Importantly, we show that the reactivation of dendrites activated during the first context exploration is sufficient to link two contextual memories. Our results demonstrate a critical role for localized dendritic plasticity in memory integration and reveal rules governing how linked and independent memories are allocated to dendritic compartments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
-
- Yokose, J. et al. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science355, 398–403 (2017). - PubMed
MeSH terms
Grants and funding
- R37 AG013622/AG/NIA NIH HHS/United States
- R01 MH124867/MH/NIMH NIH HHS/United States
- MH113071/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 AG013622/AG/NIA NIH HHS/United States
- RF1 AG013622/AG/NIA NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- R01MH124867-01/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 EY023871/EY/NEI NIH HHS/United States
- AG013622/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 MH113071/MH/NIMH NIH HHS/United States
- U01 AG013622/AG/NIA NIH HHS/United States

