Patterns of clinical response in patients with alopecia areata treated with ritlecitinib in the ALLEGRO clinical development programme
- PMID: 39962358
- PMCID: PMC12105426
- DOI: 10.1111/jdv.20547
Patterns of clinical response in patients with alopecia areata treated with ritlecitinib in the ALLEGRO clinical development programme
Abstract
Background: Ritlecitinib, an oral JAK3/TEC family kinase inhibitor, demonstrated efficacy over 48 weeks in patients with alopecia areata (AA) in the ALLEGRO phase 2b/3 study.
Objectives: This post hoc analysis evaluated individual Severity of Alopecia Tool (SALT) score trajectories in patients who received ritlecitinib 50 mg and rolled over from Phase 2b/3 into the ongoing, open-label, Phase 3 ALLEGRO-LT study to describe long-term response patterns and associated baseline disease characteristics.
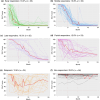
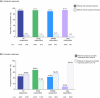
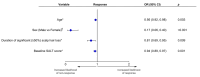
Methods: Patients aged ≥12 years with ≥50% scalp hair loss received ritlecitinib 50 mg once daily in both studies. SALT score trajectories from baseline to Month 24 were used to categorise patients as early (SALT score ≤20 at Week 24 and Months 12 and 24), middle (≤20 at Months 12 and 24) or late responders (≤20 by Month 24) or as partial responders (maintained 30% improvement), relapsers (achieved but did not maintain 30% improvement) or non-responders (did not achieve 30% improvement). The proportions of patients achieving sustained response (achieved and maintained SALT score ≤20 at all subsequent available time points through Month 24) and complete response (SALT score 0 at ≥1 time point through Month 24) were evaluated. Multivariable logistic regression assessed variables associated with response.
Results: Of 191 patients treated with ritlecitinib 50 mg, 87 (45.5%) were responders (SALT score ≤20), 24 (12.6%) were partial responders, 24 (12.6%) were relapsers and 56 (29.3%) were non-responders. Of 87 patients categorised as responders, 81 (93.1%) sustained their clinical response and 47 (46.0%) achieved complete response. Factors associated with treatment response included female sex and less extensive and shorter duration of hair loss.
Conclusions: Approximately 45% of patients were SALT score responders, with up to 11% requiring >1 year of ritlecitinib treatment to achieve response, highlighting the importance of extended treatment duration.
Gov registration: ALLEGRO phase 2b/3 study (NCT03732807); ALLEGRO-LT study (NCT04006457).
© 2025 Pfizer Inc and The Author(s). Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
BK has served on advisory boards, is a consultant, is a clinical trial investigator and/or is on a data monitoring committee for AbbVie, AltruBio, Inc., Almirall, AnaptysBio, Arena Pharmaceuticals, Aslan Pharmaceuticals, Bioniz Therapeutics, Bristol Meyers Squibb, Concert Pharmaceuticals, Inc., Equillium, Horizon Therapeutics, Eli Lilly and Company, Incyte Corp, Janssen Pharmaceuticals, LEO Pharma, Merck, Otsuka/Visterra, Inc., Pfizer, Inc., Q32 Bio, Inc., Regeneron, Sanofi Genzyme, Sun Pharmaceutical, TWi Biotechnology, Inc., Viela Bio and Ventyx Biosciences, Inc.; and has served on speakers bureaus for AbbVie, Incyte, Eli Lilly, Pfizer, Regeneron and Sanofi Genzyme. PM received investigator grants and/or research funding from Concert Pharmaceuticals, Pfizer, Inc. and Eli Lilly. KL served as an investigator, advisory board member and consultant for Pfizer, received educational grant funding from Pfizer and has been a consultant for Aquis. YR served as consultant, speaker, or investigator for AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Pfizer, Pepticom, Novartis, Janssen, Neopharm, Giuliani S.p.A, Dexcel Pharma, Eli Lilly, Sanofi, Taro and Monasterium Laboratory; and serves as the CMO of MII Labs. RS has provided professional services to Aerotech, AbbVie, AstraZeneca, Akesobio, Amgen, Arcutis, Arena, Ascend, Bayer, BMS, Boehringer Ingelheim, Celgene, Coherus BioSciences, Connect, Cutanea, Demira, Eli Lilly, Galderma, GSK, Janssen, LEO Pharma, MedImmune, Merck, MSD, Novartis, Oncobiologics, Pfizer, Regeneron, Reistone, Roche, Samson Clinical, Sanofi, Sun Pharma and UCB. LA has served as a consultant for AbbVie and L'Oreal. KE has served as a consultant for and/or received investigator grants from Abbvie, Almirall, Incyte, L'Oreal, La Roche Posay, Pfizer, Pierre Fabre, Sanofi and Viela Bio. CP has served as a consultant for Almirall, Amgen, AbbVie, Apogee Therapeutics, BMS, Boehringer Ingelheim, Celgene, Galderma, GSK, Eli Lilly, IQVIA, Janssen, Leo Pharma, Merck, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi and UCB Pharma. MO is a medical advisor for and receives advisory fees from Pfizer Japan, Inc., Taisho Pharmaceutical Co, Eli Lilly Japan KK, ROHTO Pharmaceutical Co, Bristol Myers Squibb Japan and AbbVie GK; receives lecture fees from Eli Lilly Japan KK and Pfizer Japan, Inc.; and receives research grants for projects not related to this study from Maruho Co, Sun Pharma Japan Ltd., Advantest Corp and Shiseido Co. RAE is an employee of Health Services Consulting Corporation and received consultancy fees from Pfizer in connection with this study. GB is an employee of Engineering Ingegneria Informatica, a paid sub‐contractor to Health Services Consulting Corporation, in conjunction with this analysis. UK, DW, RIA, SHZ and AL are employees of and hold stock or stock options in Pfizer, Inc.
Figures

References
-
- Islam N, Leung PS, Huntley AC, Gershwin ME. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14(2):81–89. - PubMed
-
- Lee HH, Gwillim E, Patel KR, Hua T, Rastogi S, Ibler E, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta‐analysis. J Am Acad Dermatol. 2020;82(3):675–682. - PubMed
-
- Cranwell WC, Lai VW, Photiou L, Meah N, Wall D, Rathnayake D, et al. Treatment of alopecia areata: an Australian expert consensus statement. Australas J Dermatol. 2019;60(2):163–170. - PubMed
-
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow‐up study of 191 patients. J Am Acad Dermatol. 2006;55(3):438–441. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

