Cachexia Alters Central Nervous System Morphology and Functionality in Cancer Patients
- PMID: 39962362
- PMCID: PMC11832348
- DOI: 10.1002/jcsm.13742
Cachexia Alters Central Nervous System Morphology and Functionality in Cancer Patients
Abstract
Background: Cachexia is a clinically challenging multifactorial and multi-organ syndrome, associated with poor outcome in cancer patients, and characterised by inflammation, wasting and loss of appetite. The syndrome leads to central nervous system (CNS) function dysregulation and to neuroinflammation; nevertheless, the mechanisms involved in human cachexia remain unclear.
Methods: We used in vivo structural and functional magnetic resonance imaging (Cohort 1), as well as postmortem neuropathological analyses (Cohort 2) in cachectic cancer (CC) patients compared to weight stable cancer (WSC) patients. Cohort 1 included treatment-naïve adults diagnosed with colorectal cancer, further divided into WSC (n = 12; 6/6 [male/female], 61.3 ± 3.89 years) and CC (n = 10; 6/4, 63.0 ± 2.74 years). Cohort 2 was composed by human postmortem cases where gastrointestinal carcinoma was the underlying cause of death (WSC n = 6; 3/3, 82.7 ± 3.33 years and CC n = 10; 5/5, 84.2 ± 2.28 years).
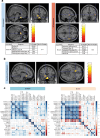
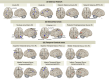
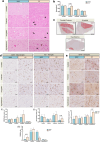
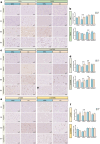
Results: Here we demonstrate that the CNS of CC patients presents regional structural differences within the grey matter (GM). Cachectic patients presented an augmented area within the region of the orbitofrontal cortex, olfactory tract and the gyrus rectus (coordinates X, Y, Z = 6, 20,-24; 311 voxels; pFWE = 0.023); increased caudate and putamen volume (-10, 20, -8; 110 voxel; pFWE = 0.005); and reduced GM in superior temporal gyrus and rolandic operculum (56,0,2; 156 voxels; pFWE = 0.010). Disrupted functional connectivity was found in several regions such as the salience network, subcortical and temporal cortical areas of cachectic patients (20 decreased and 5 increased regions connectivity pattern, pFDR < 0.05). Postmortem neuropathological analyses identified abnormal neuronal morphology and density, increased microglia/macrophage burden, astrocyte profile disruption and mTOR pathway related neuroinflammation (p < 0.05).
Conclusions: Our results indicate that cachexia compromises CNS morphology mostly causing changes in the GM of cachectic patients, leading to alterations in regional volume patterns, functional connectivity, neuronal morphology, neuroglia profile and inducing neuroinflammation, all of which may contribute to the loss of homeostasis control and to deficient information processing, as well as to the metabolic and behavioural derangements commonly observed in human cachexia. This first human mapping of CNS cachexia responses will now pave the way to mechanistically interrogate these pathways in terms of their therapeutic potential.
Keywords: central nervous system; grey matter; human cachexia; neuroimaging; neuroinflammation; neuropathology.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

