Safety and feasibility of deep brain stimulation of the anterior cingulate and thalamus in chronic refractory neuropathic pain: a pilot and randomized study
- PMID: 39962366
- PMCID: PMC11834684
- DOI: 10.1186/s10194-025-01967-8
Safety and feasibility of deep brain stimulation of the anterior cingulate and thalamus in chronic refractory neuropathic pain: a pilot and randomized study
Abstract
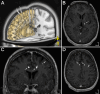
Background: Deep Brain Stimulation (DBS) of the anterior cingulum has been recently proposed to treat refractory chronic pain but its safety and its efficacy have not been evaluated in controlled conditions. Our objective was to evaluate the respective feasibility and safety of sensory thalamus (Thal-DBS) combined with anterior cingulate (ACC-DBS) DBS in patients suffering from chronic neuropathic pain.
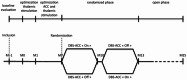
Methods: We conducted a bicentric study (clinicaltrials.gov NCT03399942) in patients suffering from medically-refractory chronic unilateral neuropathic pain surgically implanted with both unilateral Thal-DBS and bilateral ACC-DBS, to evaluate successively: Thal-DBS only; combined Thal-DBS and ACC-DBS; ACC-DBS "on" and "off" stimulation periods in randomized cross-over double-blinded conditions; and a 1-year open phase. Safety and efficacy were evaluated by repeated neurological examination, psychiatric assessment, comprehensive assessment of cognitive and affective functioning. Changes on pain intensity (Visual Analogic Scale) and quality of life (EQ-5D scale) were used to evaluate DBS efficacy.
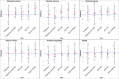
Results: All the patients (2 women, 6 men, mean age 52,1) completed the study. Adverse events were: epileptic seizure (2), transient motor or attention (2), persistent gait disturbances (1), sleep disturbances (1). No patient displayed significant cognitive or affective change. Compared to baseline, the quality of life (EQ-5D utility score) was significantly improved during the ACC-DBS "On" stimulation period (p = 0,039) and at the end of the study (p = 0,034).
Conclusion: This pilot study confirmed the safety of anterior cingulate DBS alone or in combination with thalamic stimulation and suggested that it might improve quality of life of patients with chronic refractory neuropathic pain.
Trial registration: The study has been registered on 20,180,117 (clinicaltrials.gov NCT03399942).
Keywords: Chronic Pain; Cingulate cortex; Deep brain stimulation; Neuropathic pain; Thalamus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by a Ethical Medical Committee (“Comité de Protection de Personnes Sud Méditerranée”, ID-IRB 2017-A00032-51) and registered (clinicaltrials.gov NCT03399942). All the patients signed an informed consent before inclusion. Safety data were supervised by an independent Data Monitoring Committee. Competing interests: This academic study has been sponsored and funded by the CHU de Nice (grant number: 16-AOIP-01). The hardware (leads and generators) was provided free of charge by Abbott which was not involved in the design and analysis of the study. DF is consultant for Medtronic, Abbott, and Boston Scientific, companies that manufacture and commercialize DBS devices, and received research grant from Medtronic and Abbott. MLM is consultant for Medtronic and received research grant from Medtronic. AL received speaker fees from Boston Scientific. AB has received consultant fees from Medtronic. Other authors declare no conflict of interest related to the study.
Figures

References
-
- Bouhassira D, Lantéri-Minet M, Attal N et al (2008) Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136:380–387. 10.1016/j.pain.2007.08.013 - PubMed
-
- Bouhassira D, Attal N (2011) Diagnosis and assessment of neuropathic pain: the saga of clinical tools. Pain 152:S74–S83. 10.1016/j.pain.2010.11.027 - PubMed
-
- Moisset X, Pagé MG, Pereira B, Choinière M (2022) Pharmacological treatments of neuropathic pain: real-life comparisons using propensity score matching. Pain 163:964–974. 10.1097/j.pain.0000000000002461 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

