The TANGO-DM randomized controlled trial study protocol: treatment outcomes for gestational diabetes diagnosed according to WHO 2013 or WHO 1999 thresholds
- PMID: 39962444
- PMCID: PMC11834261
- DOI: 10.1186/s12884-025-07230-x
The TANGO-DM randomized controlled trial study protocol: treatment outcomes for gestational diabetes diagnosed according to WHO 2013 or WHO 1999 thresholds
Abstract
Introduction: Gestational diabetes mellitus (GDM), or hyperglycemia first diagnosed in pregnancy, affects 7-10% of all pregnancies worldwide. Perinatal risk rises with increasing glycemia at oral glucose tolerance test (OGTT). The new (2013) WHO criteria recommend a lower fasting, and a higher post-load threshold for GDM diagnosis in comparison to the old (1999) WHO criteria. To date, however, outcomes of GDM treatment for those affected by the altered diagnostic criteria, has not been well investigated. We hypothesized that intensive GDM treatment according to the new (2013) GDM criteria would result in a reduction in infants with birth weight > 90th centile (large for gestational age, LGA), in comparison to treatment according to the old criteria (1999).
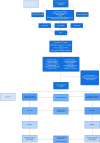
Methods: The TANGO-DM trial is an open label, multicenter randomized controlled trial. Participants are pregnant with a gestational age between 16 + 0 and 32 + 0 weeks, who underwent a 1-step venous 2- or 3-point 75-gram oral OGTT, were eligible if they had glucose concentrations discordant between the old (1999) and the new (2013) criteria. After informed consent, women are randomized to either intensive GDM treatment, consisting of dietary advice and glucose monitoring and, if euglycemia is not reached, antihyperglycemic agents, or normal obstetric care without GDM treatment. The primary outcome is large-for-gestational-age infants (birth weight > 90th percentile). Secondary outcome measures include maternal complications, obstetric complications, neonatal complications, obstetric interventions, quality of life, and healthcare and societal costs. Outcomes will be analyzed according to the intention-to-treat principle. The study is powered to detect a reduction in LGA from 16% in the untreated to 10% in the treated group, which requires 1032 participants (516 per arm; alpha-error 5% for 80% power).
Discussion: The TANGO-DM trial will provide high-level evidence to support or refute the use of the new 2013 WHO diagnostic criteria in terms of their ability to lower the number of large for gestational age infants and/or improve maternal and perinatal outcomes and/or costs in women with gestational diabetes.
Trial registration: Central Committee on Research Involving Human Subjects (CCMO) (NL63013.018.18). Registered on 22 September 2018.
Keywords: Gestational diabetes mellitus; IADPSG; Large-for-gestational-age; Randomized controlled trial; WHO 1999.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The trial has been approved by the Medical Research Ethics Committee of the Amsterdam UMC, location AMC (METC 2018_173) as well as the individual ethics boards at each center. Each participant will sign a consent form prior to participating in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, Knowler WC. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes. 2006;55(2):460–5. - PubMed
-
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian Carbohydrate Intolerance Study in Pregnant Women Trial G. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources