Cold storage of human precision-cut lung slices in TiProtec preserves cellular composition and transcriptional responses and enables on-demand mechanistic studies
- PMID: 39962456
- PMCID: PMC11834602
- DOI: 10.1186/s12931-025-03132-w
Cold storage of human precision-cut lung slices in TiProtec preserves cellular composition and transcriptional responses and enables on-demand mechanistic studies
Abstract
Background: Human precision-cut lung slices (hPCLS) are a unique platform for functional, mechanistic, and drug discovery studies in the field of respiratory research. However, tissue availability, generation, and cultivation time represent important challenges for their usage. Therefore, the present study evaluated the efficacy of a specifically designed tissue preservation solution, TiProtec, complete or in absence (-) of iron chelators, for long-term cold storage of hPCLS.
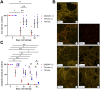
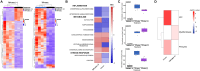
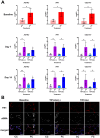
Methods: hPCLS were generated from peritumor control tissues and stored in DMEM/F-12, TiProtec, or TiProtec (-) for up to 28 days. Viability, metabolic activity, and tissue structure were determined. Moreover, bulk-RNA sequencing was used to study transcriptional changes, regulated signaling pathways, and cellular composition after cold storage. Induction of cold storage-associated senescence was determined by transcriptomics and immunofluorescence (IF). Finally, cold-stored hPCLS were exposed to a fibrotic cocktail and early fibrotic changes were assessed by RT-qPCR and IF.
Results: Here, we found that TiProtec preserves the viability, metabolic activity, transcriptional profile, as well as cellular composition of hPCLS for up to 14 days. Cold storage did not significantly induce cellular senescence in hPCLS. Moreover, TiProtec downregulated pathways associated with cell death, inflammation, and hypoxia while activating pathways protective against oxidative stress. Cold-stored hPCLS remained responsive to fibrotic stimuli and upregulated extracellular matrix-related genes such as fibronectin and collagen 1 as well as alpha-smooth muscle actin, a marker for myofibroblasts.
Conclusions: Optimized long-term cold storage of hPCLS preserves their viability, metabolic activity, transcriptional profile, and cellular composition for up to 14 days, specifically in TiProtec. Finally, our study demonstrated that cold-stored hPCLS can be used for on-demand mechanistic studies relevant for respiratory research.
Keywords: 3R; Fibrosis; Human lung models; Human precision-cut lung slices (hPCLS); Long-term cold storage; TiProtec; Tissue preservation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Institutional review board statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee of the Ludwig-Maximilians University of Munich, Germany (Ethic vote 19–630). Informed consent statement: Informed consent was obtained from all subjects involved in the study. Consent to publish: Not Applicable. Competing interests: U.R. is one of the inventors of TiProtec. She is stated as one of the inventors in the patent on this preservation solution, but the patent is held by Dr. Franz Köhler Chemie. The other authors declare no conflicts of interest.
Figures


Similar articles
-
The Fibrotic Phenotype of Human Precision-Cut Lung Slices Is Maintained after Cryopreservation.Toxics. 2024 Aug 30;12(9):637. doi: 10.3390/toxics12090637. Toxics. 2024. PMID: 39330565 Free PMC article.
-
Optimization of long-term cold storage of rat precision-cut lung slices with a tissue preservation solution.Am J Physiol Lung Cell Mol Physiol. 2021 Dec 1;321(6):L1023-L1035. doi: 10.1152/ajplung.00076.2021. Epub 2021 Oct 13. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34643087
-
Preservation of endothelial vascular function of saphenous vein grafts after long-time storage with a recently developed potassium-chloride and N-acetylhistidine enriched storage solution.Thorac Cardiovasc Surg. 2013 Dec;61(8):656-62. doi: 10.1055/s-0032-1311549. Epub 2012 Jul 12. Thorac Cardiovasc Surg. 2013. PMID: 22791204
-
Cryopreserved human precision-cut lung slices provide an immune competent pulmonary test system for "on-demand" use and long-term cultures.Toxicol Sci. 2023 Feb 17;191(2):253-265. doi: 10.1093/toxsci/kfac136. Toxicol Sci. 2023. PMID: 36617185 Free PMC article.
-
TiProtec preserves endothelial function in a rat model.J Surg Res. 2016 Jan;200(1):346-55. doi: 10.1016/j.jss.2015.06.062. Epub 2015 Jul 2. J Surg Res. 2016. PMID: 26219206
Cited by
-
Primary cell culture systems to investigate host-pathogen interactions in bacterial respiratory tract infections of livestock.Front Cell Infect Microbiol. 2025 May 9;15:1565513. doi: 10.3389/fcimb.2025.1565513. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40415959 Free PMC article. Review.
References
-
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020 A.D. 8:585–596. 10.1016/S2213-2600(20)30105-3. Cited in: PMID: 32526187. - PMC - PubMed
-
- Alsafadi HN, Staab-Weijnitz CA, Lehmann M, Lindner M, Peschel B, Königshoff M, Wagner DE. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol. 2017 A.D. 312:L896-L902. 10.1152/ajplung.00084.2017. Cited in: PMID: 28314802. - PubMed
-
- Wohlsen A, Martin C, Vollmer E, Branscheid D, Magnussen H, Becker WM, Lepp U, Uhlig S. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J. 2003 A.D. 21:1024–1032. 10.1183/09031936.03.00027502. Cited in: PMID: 12797499. - PubMed
-
- Lehmann M, Krishnan R, Sucre J, Kulkarni HS, Pineda RH, Anderson C, Banovich NE, Behrsing HP, Dean CH, Haak A et al. Precision Cut Lung Slices: Emerging Tools for Preclinical and Translational Lung Research. An Official American Thoracic Society Workshop Report. Am J Respir Cell Mol Biol. Epub ahead of print. 10.1165/rcmb.2024-0479ST. Cited in: PMID: 39499861. - PMC - PubMed
-
- Gölitz F, Herbert J, Worek F, Wille T. AChE reactivation in precision-cut lung slices following organophosphorus compound poisoning. Toxicol Lett. 2024 A.D. 392:75–83. 10.1016/j.toxlet.2023.12.014. Cited in: PMID: 38160862. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

