RGD hydrogel-loaded ADSC extracellular vesicles mitigate uranium-induced renal injury via TLR4/NF-κB pathway inhibition
- PMID: 39962465
- PMCID: PMC11834392
- DOI: 10.1186/s12951-025-03176-6
RGD hydrogel-loaded ADSC extracellular vesicles mitigate uranium-induced renal injury via TLR4/NF-κB pathway inhibition
Abstract
Background: Uranium-induced kidney damage represents a major health concern due to its toxic effects, including mitochondrial dysfunction and inflammation. Mitochondrial DNA (mtDNA)-mediated pyroptosis is a critical pathway in the pathogenesis of renal injury. The toll-like receptor 4 / nuclear factor-kappa B (TLR4/NF-κB) signaling pathway plays a pivotal role in this process. Recent studies have shown that extracellular vesicles derived from adipose-derived stem cells (ADSCs-EVs) possess therapeutic potential due to their anti-inflammatory and regenerative properties. Incorporating ADSCs-EVs into arginine-glycine-aspartate (RGD), hydrogels may enhance their stability and therapeutic efficacy in vivo. This study aims explore the molecular mechanism by which RGD hydrogel-loaded ADSCs-EVs modulate mtDNA-mediated pyroptosis by suppressing the TLR4/NF-κB signaling pathway to alleviate uranium-induced kidney injury.
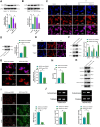
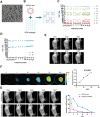
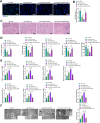
Results: Repairing mitochondrial dysfunction was found to mitigate mtDNA leakage, thereby inhibiting renal pyroptosis. ADSCs-EVs alleviated uranium-induced renal cell damage by suppressing the TLR4/NF-κB signaling pathway. In vivo animal experiments confirmed that RGD hydrogel-loaded ADSCs-EVs enhanced their stability in the body and improved their therapeutic efficacy against kidney injury.
Conclusion: Our findings reveal that RGD hydrogel-loaded ADSCs-EVs effectively inhibit the TLR4/NF-κB signaling pathway, preventing mtDNA-mediated pyroptosis and alleviating uranium-induced kidney damage. This elucidation provides a novel strategy for utilizing RGD hydrogel-loaded ADSCs-EVs in treating kidney injury.
Keywords: ADSCs-EVs extracellular vesicles; Kidney injury; Mitochondrial DNA; Pyroptosis; RGD hydrogel; TLR4/NF-κB signaling pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All animal experiments were approved by our hospital’s animal ethics committee (No. 22-07-127) and conformed to ethical norms. Competing interests: The authors declare no competing interests.
Figures






References
-
- Ortega-Romero M, Jiménez-Córdova MI, Barrera-Hernández Á, et al. Relationship between urinary biomarkers of early kidney damage and exposure to inorganic toxins in a pediatric population of Apizaco, Tlaxcala, Mexico. J Nephrol. 2023;36(5):1383–93. 10.1007/s40620-023-01598-9. - PubMed
-
- Al-Full ZZ, Khattab MR. Uranium isotopic ratio of black shale and its role in detection of oxic-anoxic conditions of uranium depositions. J Environ Sci Health Tox Hazard Subst Environ Eng. 2022;57(5):376–85. 10.1080/10934529.2022.2068886. - PubMed
-
- Xue XM, Wu XY, Zhan JM. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2023;41(11):864–9. 10.3760/cma.j.cn121094-20221209-00589 - PubMed
-
- Su SH, Su SJ, Huang LY, Chiang YC. Leukemic cells resist lysosomal inhibition through the mitochondria-dependent reduction of intracellular pH and oxidants. Free Radic Biol Med. 2023;198:1–11. 10.1016/j.freeradbiomed.2023.01.025. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

