Intraperitoneal administration of mRNA encoding interleukin-12 for immunotherapy in peritoneal carcinomatosis
- PMID: 39962479
- PMCID: PMC11834514
- DOI: 10.1186/s12951-025-03196-2
Intraperitoneal administration of mRNA encoding interleukin-12 for immunotherapy in peritoneal carcinomatosis
Abstract
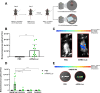
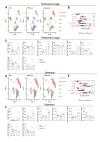
Peritoneal carcinomatosis is an advanced stage of cancer with very limited treatment options. Locoregional immunotherapy is being evaluated as a way to improve efficacy and limit toxicity. This study assessed the efficacy of a cationic polymer/lipid-based transfection compound in delivering mRNA molecules intraperitoneally. Our investigation of the transfer of luciferase mRNA in murine models of peritoneal carcinomatosis revealed preferential luciferase expression in the omentum upon the intraperitoneal administration of complexed mRNAs. Macrophages were identified as key cells that capture and express the mRNA complexes, and accordingly, depletion of resident macrophages led to reduced reporter luciferase expression. To explore the therapeutic potential of this approach, mRNA complexes encoding single-chain interleukin-12 (IL12), an immunostimulatory molecule (mRNA-IL12), were investigated. mRNA-IL12-treated mice exhibited a significant survival advantage in models of peritoneal carcinomatosis and acquired immune memory, as shown upon subcutaneous rechallenge. Tumor microenvironment analyses revealed increased numbers of CD4+ and CD8+ T cells with a more proliferative phenotype, accompanied by decreased myeloid populations in the omentum. Overall, our study underscores the potential of mRNA complexes for efficient mRNA delivery, eliciting effective antitumor responses and modulating the tumor microenvironment to treat peritoneal carcinomatosis.
Keywords: Cancer immunotherapy; Interleukin-12; Locoregional treatment; Peritoneal carcinomatosis; mRNA.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All the experiments received approval (R-080–19GN) from the Ethics Committee for Animal Testing at the University of Navarra. Consent for publication: Not applicable. Competing interests: Ignacio Melero reports receiving commercial research grants from AstraZeneca, BMS, Highlight Therapeutics, Alligator, Pfizer Genmab and Roche; has received speakers bureau honoraria from MSD; and is a consultant or advisory board member for BMS, Roche, AstraZeneca, Genmab, Pharmamar, F-Star, Bioncotech, Bayer, Numab, Pieris, Gossamer, Alligator and Merck Seron.The rest of the authors report no conflicts of interest in this work.
Figures



References
-
- Aranda F, Eguren-Santamaria I, Bella A, Berraondo P, Melero I. Revisiting Intracavitary Immunotherapy of Cancer. Clin Cancer Res May. 2022;13(10):1993–5. 10.1158/1078-0432.CCR-22-0201 - PubMed
-
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol Oct. 2003;15(20):3737–43. 10.1200/JCO.2003.04.187 - PubMed
-
- Cortes-Guiral D, Hubner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers Dec. 2021;16(1):91. 10.1038/s41572-021-00326-6 - PubMed
MeSH terms
Substances
Grants and funding
- FPU21/00042/FPU grant from The Spanish Ministry of Education and Professional training
- CP19/00114/ISCIII (Instituto de Salud Carlos III), cofinanced by FSE (Fondo Social Europeo)
- PI20/00203 and PI22/00147/Instituto de Salud Carlos III and co-financed by the European Union
- 0011-1411-2023/Gobierno de Navarra Proyecto ARNMUNE
- 2022/Leonardo Grant for Researchers and Cultural Creators (BBVA Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

