Crosstalk between renin and arachidonic acid (and its metabolites)
- PMID: 39962508
- PMCID: PMC11831833
- DOI: 10.1186/s12944-025-02463-3
Crosstalk between renin and arachidonic acid (and its metabolites)
Abstract
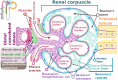
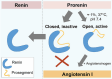
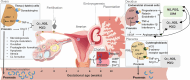
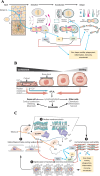
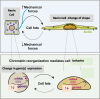
Renin plays a significant role in the regulation of blood pressure and fluid volume by modulating the renin‒angiotensin‒aldosterone (RAAS) system. Renin suppression reduces serum aldosterone levels and lowers blood pressure in addition to preserving renal function. However, exactly how renin synthesis and action are regulated and how renin suppression preserves renal function are not clear. We propose that arachidonic acid (AA) and its metabolites control renin synthesis, secretion, and action by virtue of its (AA) anti-inflammatory, cytoprotective actions and ability to regulate the secretion of renin. These findings suggest that direct renin suppression results in changes in AA metabolism. This proposal implies that AA and its metabolites may be developed as potential drugs to prevent and manage hypertension and preserve renal function.
Keywords: Arachidonic acid; Inflammation; Neurotransmitters; Nitric oxide; Renin; Renin-angiotensin-aldosterone system.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures






References
-
- Ueno M, Fujii W, Ono W, Murata H, Fujigaki Y, Shibata S. Renin inhibition and the long-term renal function in patients with 3 hypertensive emergency: a retrospective cohort study. Am J HTN, in press. - PubMed
-
- Heesch CM. Reflexes that Control Cardiovascular function. Am J Physiol Content. 1999;277:S234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

