Unilateral and Bilateral Subthalamic Deep Brain Stimulation Differently Favour Apathy in Parkinson's Disease
- PMID: 39962903
- PMCID: PMC11833280
- DOI: 10.1111/ejn.70019
Unilateral and Bilateral Subthalamic Deep Brain Stimulation Differently Favour Apathy in Parkinson's Disease
Abstract
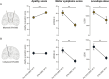
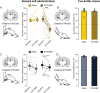
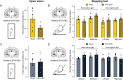
The link between subthalamic nucleus deep brain stimulation (STN-DBS) and apathy in patients with Parkinson's disease (PD) remains a controversial topic. The literature is mixed and the most supported explanation is the reduction of dopaminergic treatment. Yet a body of clinical and experimental evidences suggest that STN-DBS itself can also promote apathy in certain patients. However, the parameters accounting for apathy heterogeneity in stimulated patients along with the mechanisms underlying apathy induced by STN-DBS remain to be investigated. Whether bilateral and unilateral STN-DBS have the same influence on apathy is for instance unknown. We previously and separately showed in patients and rodents that bilateral STN-DBS can promote apathy per se. Here, we compare the effect of bilateral versus unilateral STN-DBS both in patients and in rodents. We conducted a clinical follow-up of patients with Parkinson's disease undergoing unilateral or bilateral STN-DBS and assessing apathy 3 months before and after STN-DBS. In parallel, we applied chronic and uninterrupted unilateral or bilateral DBS in rodents and extract longitudinal motivational changes with a battery of behavioural tests. While bilateral STN-DBS promotes apathy in patients and induces a loss of motivation in rodents, we found that unilateral STN-DBS did not exert such an effect both in patients and in rats. These data show that bilateral but not unilateral STN-DBS promotes apathy. This not only substantiate the induction of neuropsychiatric effects by STN-DBS but also suggest that this might be circumvented if STN-DBS is applied unilaterally instead of bilaterally.
Keywords: Parkinson's disease; apathy; motivation; subthalamic nucleus deep brain stimulation.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Aarsland, D. , Bronnick K., Alves G., et al. 2009. “The Spectrum of Neuropsychiatric Symptoms in Patients With Early Untreated Parkinson's Disease.” Journal of Neurology, Neurosurgery, and Psychiatry 80: 928–930. - PubMed
-
- Baunez, C. , Christakou A., Chudasama Y., Forni C., and Robbins T. W.. 2007. “Bilateral High‐Frequency Stimulation of the Subthalamic Nucleus on Attentional Performance: Transient Deleterious Effects and Enhanced Motivation in Both Intact and Parkinsonian Rats.” European Journal of Neuroscience 25: 1187–1194. - PMC - PubMed
-
- Benabid, A. L. , Chabardes S., Mitrofanis J., and Pollak P.. 2009. “Deep Brain Stimulation of the Subthalamic Nucleus for the Treatment of Parkinson's Disease.” Lancet Neurology 8: 67–81. - PubMed
-
- Benabid, A. L. , Krack P. P., Benazzouz A., Limousin P., Koudsie A., and Pollak P.. 2000. “Deep Brain Stimulation of the Subthalamic Nucleus for Parkinson's Disease: Methodologic Aspects and Clinical Criteria.” Neurology 55: S40–S44. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

