Blood biomarker dynamics in people with relapsing multiple sclerosis treated with cladribine tablets: results of the 2-year MAGNIFY-MS study
- PMID: 39963134
- PMCID: PMC11830603
- DOI: 10.3389/fimmu.2025.1512189
Blood biomarker dynamics in people with relapsing multiple sclerosis treated with cladribine tablets: results of the 2-year MAGNIFY-MS study
Erratum in
-
Corrigendum: Blood biomarker dynamics in people with relapsing multiple sclerosis treated with cladribine tablets: results of the 2-year MAGNIFY-MS study.Front Immunol. 2025 Mar 5;16:1571978. doi: 10.3389/fimmu.2025.1571978. eCollection 2025. Front Immunol. 2025. PMID: 40109340 Free PMC article.
Abstract
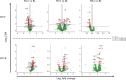
Background and objectives: Cladribine tablets (CladT) represent an effective immune reconstitution therapy, administered in short treatment courses over two consecutive years. To better understand the amplitude of immune changes, we performed a comprehensive analysis during the 2-year study period for the entire MAGNIFY-MS population (N=270). In addition to lymphocyte kinetics, we studied intracellular cytokines serum proteins, and their associations with clinical outcomes. To put these changes into perspective, we analyzed transcriptional changes in T and B cells and associated biological pathways before and after each treatment course with CladT.
Methods: Immunophenotyping and transcriptomics were performed at regular visits with major differences reported between baseline (BL) and after each yearly treatment course. Assessments included: lymphocyte dynamics, RNA sequencing (B and T cells), intracellular cytokines, serum proteins (immunoglobulins [IgG and IgM], and serum neurofilament light chain [sNfL]). Clinical measures included: MRI activity, annualized relapse rate (ARR), 6-month confirmed disability progression (6mCDP), timed 25-foot walk (T25FW), and 9-hole peg test (9HPT).
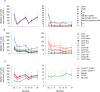
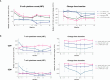
Results: All B, T and NK cells were reduced at month (M)3 after CladT administration, except regulatory B cells which increased above BL from M3 to M24. Naïve and transitional B cells recovered at M6; all other B and T cell subsets remained below BL levels. Reductions in all NK cell subtypes were observed at M3, CD16lowCD56bright and NKp46 cells reconstituted at M6 and M12 respectively. Changes in genes and pathways associated with innate and adaptive immune response were observed after CladT treatment, along with reductions in pro-inflammatory cytokine-producing B and T cells and increases in anti-inflammatory cytokine-producing T cells. IgG and IgM levels remained above the lower limits of normal in most participants. sNfL levels decreased, remaining reduced by M24. Significant reductions in the annualized combined unique active lesion count occurred from M2 onwards. ARR was 0.11 (95% confidence interval: 0.09,0.15), with 83% participants free of qualifying relapses. Over 90% of participants were free of 6mCDP, around 87% had no confirmed progression on T25FW and 9HPT. No significant correlations were seen between clinical parameters and lymphocyte dynamics to M6. The safety profile was consistent with previous reports.
Discussion: Deep longitudinal immunophenotyping, analysis of transcriptional changes, reduction in cells expressing pro-inflammatory cytokines, along with the marker of neuroaxonal damage provide novel and innovative evidence of CladT rebalancing the immune system towards a more homeostatic and less pathogenic state.
Clinical trial registration: https://clinicaltrials.gov/study/, identifier NCT03364036.
Keywords: biomarkers; cladribine tablets; immune reconstitution therapy; multiple sclerosis; transcriptomics, immunophenotyping.
Copyright © 2025 Wiendl, Barkhof, Montalban, Achiron, Derfuss, Chan, Hodgkinson, Prat, Leocani, Schmierer, Sellebjerg, Vermersch, Jin, Chudecka, Kloetgen, Lin, Gardner and De Stefano.
Conflict of interest statement
HW is member of scientific advisory boards/steering committees for Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. He received speaker honoraria and travel support from Bayer, Biogen, CSL Behring, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Fresenius Medical Care, Merck, Omniamed, Novartis, Sanofi, and Teva. He received compensation as a consultant from Biogen, Merck Novartis, Omniamed, Roche, and Sanofi. He has received research support from Bayer, Biogen, Merck, Novartis, Sanofi, and Teva, as well as the German Ministry for Education and Research BMBF, German Research Foundation DFG, Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, Merck, Novartis, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies IZKF Münster, and RE Children’s Foundation. FB is supported by the NIHR Biomedical Research Centre at UCLH and is a steering committee or Data Safety Monitoring Board member for ATRI/ACTC, Biogen, Merck and Prothena. Consultant for Celltrion, Combinostics, IXICO, Janssen, Merck, Rewind Therapeutics, Roche. Research agreements with Biogen, GE Healthcare, Merck Roche. Co-founder and shareholder of Queen Square Analytics Ltd. XM has received compensation for lecture honoraria and travel expenses, participation in scientific meetings, clinical trial steering committee membership, or clinical advisory board participation in recent years from Abbvie, Actelion, Alexion, Bial PD, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Genzyme, Hoffmann-La Roche, Immunic Therapeutics, Janssen Pharmaceuticals, Medday, Medscape, Merck, Mylan, Nervgen, Neuraxpharm, Novartis, Peervoice, Samsung-Biosys, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, ECTRIMS, MSIF, and NMSS or any of their affiliates. AA has received over the last 5 years honoraria or consulting fees for participating in advisory boards related to clinical trial design, trial steering committees, and data and safety monitoring committees from Biogen, Bristol Myers Squibb, Merck Novartis, Roche, and Sanofi; and research support for investigator-initiated trials and MS patients’ benefits activities from Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, and Sanofi. TD serves on scientific advisory boards for Actelion Janssen/J&J, Bayer, Biogen, Celgene BMS, GeNeuro, MedDay, Merck, Mitsubishi Pharma, Novartis, Roche, and Sanofi; has received funding for travel and/or speaker honoraria from Biogen, Merck Novartis, Roche, and Sanofi; and receives research support from Actelion, the European Union, Novartis, Roche, the Swiss MS Society, and the Swiss National Foundation. ACha has received speakers’/board honoraria from Actelion Janssen/J&J, Almirall, Bayer, Biogen, Celgene BMS, Merck, Novartis, Roche, Sanofi, and Teva, all for hospital research funds. He received research support from Biogen, CSL Behring, Sanofi, and UCB, the European Union, and the Swiss National Foundation. He serves as associate editor of the European Journal of Neurology, on the editorial board for Clinical and Translational Neuroscience, and as topic editor for the Journal of International Medical Research. SH serves on advisory boards for Bayer, Biogen, Merck Novartis, Roche, and Sanofi. She has received money for travel and speaker honoraria from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. AP has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years, and/or received operating grants from Alexion, Bayer, Biogen, Celgene Bristol Myers Squibb, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Novartis, Roche, Sanofi, and Teva. LL has received honoraria for consulting services or speaking activities from Merck Novartis, and Roche; and research support from Merck and Novartis. KS has received research support, through Queen Mary University of London, from Biogen, Merck, Novartis, and Sandoz; speaking honoraria from, and/or served in an advisory role for, Biogen, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Merck, Neuraxpharm, Novartis, Roche, and Sanofi; and remuneration for teaching activities from AcadeMe and Medscape. FS has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria, or received research support for his laboratory from Biogen, Celgene BMS, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA Merck Novartis, Roche, Sanofi, and Teva. PV has received honoraria or consulting fees from AB Science, Ad Scientiam, Biogen, Celgene BMS, Imcyse, Merck, Novartis, Roche, Sanofi, and Teva; and research support from Novartis, Roche, and Sanofi. HJ and AK are employees of Merck Healthcare KGaA, Darmstadt, Germany. AChu is an employee of Cytel Inc., Geneva Branch, Switzerland, funded by to perform statistical analyses for this study. LG and DL are employees of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. NDS has received honoraria from Biogen, Celgene Bristol Myers Squibb, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. Genzyme, Immunic, Novartis, Roche, and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Biogen, Genzyme, Immunic, Merck, Novartis, and Roche, and has received research grant support from the Italian MS Society.
Figures


References
-
- Comi G, Cook S, Giovannoni G, Rieckmann P, Soelberg Sørensen P, Vermersch P, et al. . Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. (2019) 29:168–74. doi: 10.1016/j.msard.2019.01.038 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

