Biodegradable Electrospun PLGA Nanofibers-Encapsulated Trichinella Spiralis Antigens Protect from Relapsing Experimental Autoimmune Encephalomyelitis and Related Gut Microbiota Dysbiosis
- PMID: 39963417
- PMCID: PMC11830953
- DOI: 10.2147/IJN.S499161
Biodegradable Electrospun PLGA Nanofibers-Encapsulated Trichinella Spiralis Antigens Protect from Relapsing Experimental Autoimmune Encephalomyelitis and Related Gut Microbiota Dysbiosis
Abstract
Purpose: Trichinella spiralis has evolved complex immunomodulatory mechanisms mediated by excretory-secretory products (ESL1) that enable its survival in the host. Consequently, ESL1 antigens display excellent potential for treating autoimmune diseases such as multiple sclerosis (MS). However, whether timely controlled delivery of ESL1 antigens in vivo, as in natural infections, could enhance its therapeutic potential for MS is still unknown.
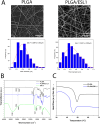
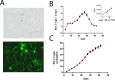
Methods: To test this, we encapsulated ESL1 antigens into biodegradable poly (lactide-co-glycolic) acid (PLGA) nanofibers by emulsion electrospinning as a delivery system and assessed their release dynamics in vitro, and in an animal MS model, experimental autoimmune encephalomyelitis (EAE), induced 7 days after PLGA/ESL1 subcutaneous implantation. PLGA/ESL1 effects on EAE symptoms were monitored along with multiple immune cell subsets in target organs at the peak and recovery of EAE. Gut barrier function and microbiota composition were analyzed using qPCR, 16S rRNA sequencing, and metabolomic analyses.
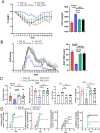
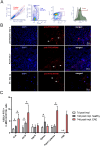
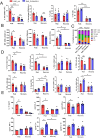
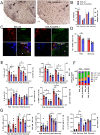
Results: ESL1 antigens, released from PLGA and drained via myeloid antigen-presenting cells through lymph nodes, protected the animals from developing EAE symptoms. These effects correlated with reduced activation of myeloid cells, increased IL-10 expression, and reduced accumulation of proinflammatory natural killer (NK) cells, T helper (Th)1 and Th17 cells in the spleen and central nervous system (CNS). Additionally, CD4+CD25hiFoxP3+ regulatory T cells and IL-10-producing B cells were expanded in PLGA/ESL1-treated animals, compared to control animals. The migration of ESL1 to the guts correlated with locally reduced inflammation and gut barrier damage. Additionally, PLGA/ESL1-treated animals displayed an unaltered microbiota characterized only by a more pronounced protective mevalonate pathway and expanded short-chain fatty acid-producing bacteria, which are known to suppress inflammation.
Conclusion: The delivery of T. spiralis ESL1 antigens via biodegradable electrospun PLGA nanofiber implants efficiently protected the animals from developing EAE by inducing a beneficial immune response in the spleen, gut, and CNS. This platform provides excellent grounds for further development of novel MS therapies.
Keywords: PLGA nanofibers; drug delivery; electrospinning; gut microbiota; immune modulation; tolerogenic cells.
© 2025 Sabljić et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Application of dendritic cells stimulated with Trichinella spiralis excretory-secretory antigens alleviates experimental autoimmune encephalomyelitis.Med Microbiol Immunol. 2013 Jun;202(3):239-49. doi: 10.1007/s00430-012-0286-6. Epub 2013 Jan 11. Med Microbiol Immunol. 2013. PMID: 23307236
-
Constipation induced gut microbiota dysbiosis exacerbates experimental autoimmune encephalomyelitis in C57BL/6 mice.J Transl Med. 2021 Jul 23;19(1):317. doi: 10.1186/s12967-021-02995-z. J Transl Med. 2021. PMID: 34301274 Free PMC article.
-
Trichinella spiralis excretory-secretory products downregulate MMP-9 in Dark Agouti rats affected by experimental autoimmune encephalomyelitis.Exp Parasitol. 2021 Jun;225:108112. doi: 10.1016/j.exppara.2021.108112. Epub 2021 May 6. Exp Parasitol. 2021. PMID: 33964315
-
Interaction of the Gut Microbiome and Immunity in Multiple Sclerosis: Impact of Diet and Immune Therapy.Int J Mol Sci. 2023 Sep 29;24(19):14756. doi: 10.3390/ijms241914756. Int J Mol Sci. 2023. PMID: 37834203 Free PMC article. Review.
-
Trichinella spiralis: inflammation modulator.J Helminthol. 2020 Sep 21;94:e193. doi: 10.1017/S0022149X20000802. J Helminthol. 2020. PMID: 32951619 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

