Role of alpha-synuclein seed amplification assay in Parkinson's disease clinical trials: A case of misdiagnosis
- PMID: 39963592
- PMCID: PMC11832018
- DOI: 10.1016/j.prdoa.2024.100274
Role of alpha-synuclein seed amplification assay in Parkinson's disease clinical trials: A case of misdiagnosis
Abstract
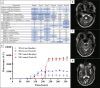
•Early differentiation between PD and atypical parkinsonism is challenging.•Variability in diagnosis can weaken statistical power in trials.•Precise diagnostic tools are needed for trial population homogeneity.•This patient initially diagnosed with PD, was diagnosed with MSA via CSF αSyn-SAA.•Adding αSyn-SAA to inclusion criteria could enhance subject selection in trials.
Keywords: Alpha-synuclein; Clinical trial; Multiple System Atrophy; Parkinson’s disease.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mya C. Schiess and Mohammad Shahnawaz have received financial support from grants provided by the Michael J. Fox Foundation (MJFF) for Parkinson’s Research. Mohammad Shahnawaz is the inventor of the patent for PMCA under #US10989718B2, licensed to the University of Texas Health Science Center at Houston and Amprion Inc. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Rizzo G., Copetti M., Arcuti S., Martino D., Fontana A., Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology. 2016;86(6):566–576. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

