Safety and Efficacy of Novel RNA Interference Therapeutic Agent Zilebesiran in People With Hypertension: A Systematic Review and Meta-Analysis
- PMID: 39963626
- PMCID: PMC11831239
- DOI: 10.7759/cureus.77607
Safety and Efficacy of Novel RNA Interference Therapeutic Agent Zilebesiran in People With Hypertension: A Systematic Review and Meta-Analysis
Abstract
Hypertension is a major risk factor for cardiovascular and renal diseases. Despite the availability of a wide variety of anti-hypertensive agents, a large number of patients on anti-hypertensive therapy have uncontrolled hypertension. Several factors, such as incorrect dosage and treatment non-adherence due to side effects, polypharmacy, and complex dosing regimens, can cause inadequate blood pressure regulation. Under phase 1 and 2 trials, Zilebesiran is a subcutaneously administered novel RNA interference therapeutic agent that reduces blood pressure by targeting angiotensinogen (AGT) synthesis. We conducted this systematic review and meta-analysis (SRMA) to evaluate the safety and efficacy of Zilebesiran in reducing blood pressure. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines, a literature search was carried out across Pubmed, Embase, and Cochrane Central databases. Our study included two randomized controlled trials (RCTs) involving adult participants receiving Zilebesiran or placebo. The primary outcomes assessed were changes in mean 24-hour ambulatory systolic and diastolic blood pressure and the incidence of adverse events at 12 weeks. Secondary outcomes included changes in serum creatinine and estimated glomerular filtration rate. Zilebesiran administration resulted in a significant reduction in 24-hour systolic blood pressure (-15.12 mmHg, 95% CI: -17.21 to -13.03) and diastolic blood pressure (-7.34 mmHg, 95% CI: -8.70 to -5.98) at 12 weeks compared to placebo with I² = 0%, indicating consistent results across trials. A statistically significant increase in total adverse events was noted (risk ratio: 1.15, 95% CI: 1.01 to 1.30), while serious adverse events did not differ significantly. The commonly encountered adverse effects included pain and erythema at the injection site and headache. Renal safety was supported by consistent serum creatinine and eGFR levels across the included studies. Zilebesiran demonstrates substantial efficacy in blood pressure reduction and exhibits a favorable safety profile, presenting a potential alternative for patients, particularly those struggling with adherence to daily oral medications. The inclusion of only two RCTS resulted in a small sample size, predominantly composed of white participants. This limits the generalizability of the results and highlights the need for further trials with a larger and more diverse study population. Further long-term investigations are also needed to establish the drug's impact on body weight, blood glucose levels, lipid profile, cardiovascular and hepatic health, and its efficacy when used alongside conventional antihypertensive medications.
Keywords: blood pressure; hypertension; raas; rna interference; systematic review and meta-analysis; zilebesiran.
Copyright © 2025, Singh et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
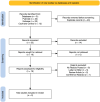

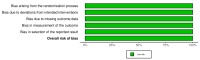




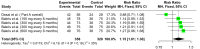

References
-
- Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. Forouzanfar MH, Liu P, Roth GA, et al. JAMA. 2017;317:165–182. - PubMed
-
- Hypertension. [ Oct; 2024 ]. 2024. https://www.who.int/news-room/fact-sheets/detail/hypertension https://www.who.int/news-room/fact-sheets/detail/hypertension
-
- Treatment of hypertension: a review. Carey RM, Moran AE, Whelton PK. JAMA. 2022;328:1849–1861. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
