Hypoxia-driven mobilization of altruistic cancer stem cells in platinum-treated head and neck cancer
- PMID: 39963656
- PMCID: PMC11830676
- DOI: 10.3389/fimmu.2024.1336882
Hypoxia-driven mobilization of altruistic cancer stem cells in platinum-treated head and neck cancer
Abstract
Background: Head and neck cancers harbor dormant cancer stem cells (CSCs). This study explores how platinum therapy impacts these cells in a non-genetic manner and the role of hypoxia in this process. Previously, we identified a novel population of CSCs exhibiting an "altruistic" phenotype, sacrificing self-renewal to promote niche defense (tumor stemness defense, TSD), potentially protecting a dormant subpopulation of CSCs, the reawakening CSC (R-CSC) retaining stress memory. This TSD phenotype involves the activation of the MYC-HIF2α pathway and, importantly, is linked to a hypoxic tumor microenvironment. We termed these TSD+ CSCs "altruistic cancer stem cells" (A-CSCs). Here we investigated the potential role of tumor hypoxia in the mobilization of TSD+ CSCs to the circulation as a part of niche defense against platinum therapy.
Methods: We isolated CTCs and primary tumor cells from head and neck squamous cell carcinoma (HNSCC) patients undergoing platinum therapy (n = 14). We analyzed the TSD phenotype and markers of hypoxia in these cells. Additionally, we further characterized a previously reported pre-clinical model of platinum-induced tumor stemness to study the link between hypoxia, TSD+ CSC emergence, and mobilization to the circulation and bone marrow.
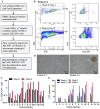
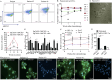
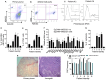
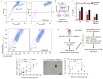
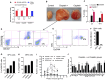
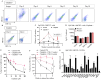
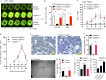
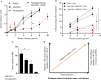
Results: We isolated TSD+ CTCs with a hypoxic signature from eight out of 14 HNSCC patients. These cells displayed increased proliferation and invasion upon cisplatin treatment, suggesting a role in niche defense. Our pre-clinical model confirmed that hypoxia directly correlates with the expansion of TSD+ CSCs and their mobilization into the circulation and bone marrow following cisplatin treatment. We demonstrated the protection of R-CSCs by TSD+ CSCs. Notably, inhibiting hypoxia alone with tirapazamine did not reduce TSD+ CSCs, CTCs, or R-CSCs. However, combining tirapazamine with FM19G11, a MYC-HIF2α pathway inhibitor, significantly reduced the platinum-induced expansion of both TSD+ CSCs, CTCs, and the presence of R-CSCs in the bone marrow.
Conclusions: This study reveals that HNSCC patients undergoing platinum therapy can harbor TSD+ CTCs exhibiting an altruistic phenotype and a hypoxic signature. Additionally, the pre-clinical study provides a novel non-genetic mechanism of therapy resistance-the altruistic tumor self-defense. The tumor microenvironment, through the emergence of TSD+ CSCs, appears to act collectively to defend the tumor self-identity by hijacking an altruistic stem cell niche defense mechanism.
Keywords: altruistic stem cells (ASCs); cancer stem cells (CSCs); circulating tumor cells; head and neck squamous cell carcinoma (HNSCC); platinum chemotherapy; reawakening CSCs (R-CSCs); tumor hypoxia; tumor stemness defense (TSD).
Copyright © 2025 Pathak, Pal, Talukdar, Saikia, Sandhya, Tasabehji, Li, Phukan, Bhuyan, Patra and Das.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

