Multiomic Analysis of Calf Muscle in Peripheral Artery Disease and Chronic Kidney Disease
- PMID: 39963788
- PMCID: PMC11949227
- DOI: 10.1161/CIRCRESAHA.124.325642
Multiomic Analysis of Calf Muscle in Peripheral Artery Disease and Chronic Kidney Disease
Abstract
Background: Chronic kidney disease (CKD) has emerged as a significant risk factor that accelerates atherosclerosis, decreases muscle function, and increases the risk of amputation or death in patients with peripheral artery disease (PAD). However, the modulators underlying this exacerbated pathobiology are ill-defined. Recent work has demonstrated that uremic toxins are associated with limb amputation in PAD and have pathological effects in both the limb muscle and vasculature. Herein, we use multiomics to identify novel modulators of disease pathobiology in patients with PAD and CKD.
Methods: A cross-sectional study enrolled 4 groups of participants: controls without PAD or CKD (n=28), patients with PAD only (n=46), patients with CKD only (n=31), and patients with both PAD and CKD (n=18). Both targeted (uremic toxins) and nontargeted metabolomics in plasma were performed using mass spectrometry. Calf muscle biopsies were used to measure histopathology, perform bulk and single-nucleus RNA sequencing, and assess mitochondrial function. Differential gene and metabolite analyses, as well as pathway and gene set enrichment analyses, were performed.
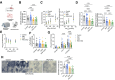
Results: Patients with both PAD and CKD exhibited significantly lower calf muscle strength and smaller muscle fiber areas compared with controls and those with only PAD. Compared with controls, mitochondrial function was impaired in patients with CKD, with or without PAD, but not in PAD patients without CKD. Plasma metabolomics revealed substantial alterations in the metabolome of patients with CKD, with significant correlations observed between uremic toxins (eg, kynurenine and indoxyl sulfate) and both muscle strength and mitochondrial function. RNA sequencing analyses identified downregulation of mitochondrial genes and pathways associated with protein translation in patients with both PAD and CKD. Single-nucleus RNA sequencing further highlighted a mitochondrial deficiency in muscle fibers along with unique remodeling of fibro-adipogenic progenitor cells in patients with both PAD and CKD, with an increase in adipogenic cell populations.
Conclusions: CKD significantly exacerbates ischemic muscle pathology in PAD, as evidenced by diminished muscle strength, reduced mitochondrial function, and altered transcriptome profiles. The correlation between uremic toxins and muscle dysfunction suggests that targeting these metabolites may offer therapeutic potential for improving muscle health in PAD patients with CKD.
Keywords: mitochondria; muscles; peripheral arterial disease; renal insufficiency, chronic; uremia.
Conflict of interest statement
None.
Figures






References
-
- Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2007;55:583–589. doi: 10.1111/j.1532-5415.2007.01123.x - PubMed
-
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. . Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 - PubMed
-
- Heideman PP, Rajebi MR, McKusick MA, Bjarnason H, Oderich GS, Friese JL, Fleming MD, Stockland AH, Harmsen WS, Mandrekar J, et al. . Impact of chronic kidney disease on clinical outcomes of endovascular treatment for femoropopliteal arterial disease. J Vasc Interv Radiol. 2016;27:1204–1214. doi: 10.1016/j.jvir.2016.04.036 - PMC - PubMed
-
- O’Hare AM, Sidawy AN, Feinglass J, Merine KM, Daley J, Khuri S, Henderson WG, Johansen KL. Influence of renal insufficiency on limb loss and mortality after initial lower extremity surgical revascularization. J Vasc Surg. 2004;39:709–716. doi: 10.1016/j.jvs.2003.11.038 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

