Acute Myocarditis and Inflammatory Cardiomyopathies: Insights From Cardiac Magnetic Resonance Findings
- PMID: 39963997
- PMCID: PMC11834149
- DOI: 10.1111/echo.70099
Acute Myocarditis and Inflammatory Cardiomyopathies: Insights From Cardiac Magnetic Resonance Findings
Abstract
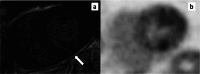
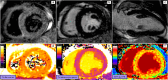
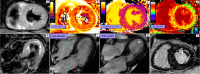
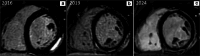
Myocardial inflammation encompasses a broad spectrum of conditions, including acute myocarditis, chronic inflammatory cardiomyopathy, and several overlapping entities that differ in clinical presentation, pathophysiology, and progression. These conditions range from self-limiting acute inflammation to chronic myocardial injury and dysfunction. The etiologic classification of myocardial inflammation highlights the complexity of its pathogenesis, involving direct tissue damage, immune-mediated mechanisms, and environmental triggers. Cardiac magnetic resonance (CMR) imaging has become a central diagnostic tool in the assessment of myocardial inflammation, providing precise characterization of myocardial tissue, assessing cardiac function, and stratifying prognosis. Advanced techniques such as T1 and T2 mapping and extracellular volume quantification have further expanded its diagnostic capabilities. This review highlights the essential role of CMR in diagnosing myocardial inflammation, recognizing various imaging findings associated with different underlying causes, and informing clinical management. The standardization of CMR protocols, along with advancements in imaging techniques and strengthened interdisciplinary collaboration, represents a fundamental step toward improving diagnostic accuracy, patient outcomes, and the understanding of the broad spectrum of myocardial inflammatory diseases.
Keywords: cardiovascular magnetic resonance; chronic inflammatory cardiomyopathy; myocardial inflammation; myocarditis.
© 2025 The Author(s). Echocardiography published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

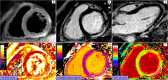
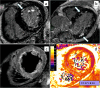
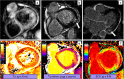
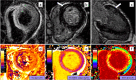
References
-
- Caforio A. L. P., Pankuweit S., Arbustini E., et al., “Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases,” European Heart Journal 34 (2013): 2636–2648. - PubMed
-
- Hufnagel G., Pankuweit S., Richter A., Schönian U., and Maisch B., “The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID),” Herz 25 (2000): 279–285. - PubMed
-
- Kociol R. D., Cooper L. T., and Fang J., “Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association,” Circulation 141 (2020): e69–e92. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

