Patchy Charge Distribution Affects the pH in Protein Solutions during Dialysis
- PMID: 39964136
- PMCID: PMC11887432
- DOI: 10.1021/acs.langmuir.4c04942
Patchy Charge Distribution Affects the pH in Protein Solutions during Dialysis
Abstract
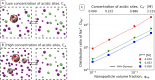
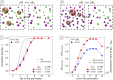
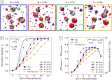
When using dialysis ultra- or diafiltration to purify protein solutions, a dialysis buffer in the permeate is employed to set the pH in the protein solution. Failure to achieve the target pH may cause undesired precipitation of the valuable product. However, the pH in the permeate differs from that in the retentate, which contains the proteins. Experimental optimization of the process conditions is time-consuming and expensive, while accurate theoretical predictions still pose a major challenge. Current models of dialysis account for the Donnan equilibrium, acid-base properties, and ion-protein interactions, but they neglect the patchy distribution of ionizable groups on the proteins and its impact on the solution properties. Here, we present a simple computational model of a colloidal particle with weakly acidic sites on the surface, organized in patches. This minimalistic model allows systematic variation of the relevant parameters, while simultaneously demonstrating the essential physics governing the acid-base equilibria in protein solutions. Using molecular simulations in the Grand-Reaction ensemble, we demonstrate that interactions between ionizable sites significantly affect the nanoparticle charge and thereby contribute to pH difference between the permeate and retentate. We show that the significance of this contribution increases if the ionizable sites are located on a smaller patch. Protein solutions are governed by the same physics as our simple model. In this context, our results show that models which aim to quantitatively predict the pH in protein solutions during dialysis need to account for the patchy distribution of ionizable sites on the protein surface.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Liderfelt J.; Royce J.. Biopharmaceutical Processing; Jagschies G.; Lindskog E.; Ła̧cki K.; Galliher P., Eds.; Elsevier, 2018; pp 441–453.
-
- Zydney A. L. New developments in membranes for bioprocessing - A review. J. Membr. Sci. 2021, 620, 118804 10.1016/j.memsci.2020.118804. - DOI
-
- Briskot T.; Hillebrandt N.; Kluters S.; Wang G.; Studts J.; Hahn T.; Huuk T.; Hubbuch J. Modeling the Gibbs-Donnan effect during ultrafiltration and diafiltration processes using the Poisson-Boltzmann theory in combination with a basic Stern model. J. Membr. Sci. 2022, 648, 120333 10.1016/j.memsci.2022.120333. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

