Identification of two glycosyltransferases required for synthesis of membrane glycolipids in Clostridioides difficile
- PMID: 39964170
- PMCID: PMC11898633
- DOI: 10.1128/mbio.03512-24
Identification of two glycosyltransferases required for synthesis of membrane glycolipids in Clostridioides difficile
Abstract
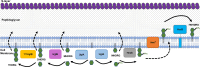
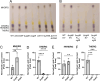
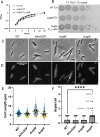
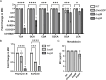
Clostridioides difficile infections cause over 12,000 deaths and an estimated one billion dollars in healthcare costs annually in the United States. The cell membrane is an essential structure that is important for protection from the extracellular environment, signal transduction, and transport of nutrients. The polar membrane lipids of C. difficile are ~50% glycolipids, a higher percentage than most other organisms. The glycolipids of C. difficile consist of monohexosyldiradylglycerol (MHDRG) (~14%), dihexosyldiradylglycerol (DHDRG) (~15%), trihexosyldiradylglycerol (THDRG) (~5%), and a unique glycolipid aminohexosyl-hexosyldiradylglycerol (HNHDRG) (~16%). Previously, we found that HexSDF are required for the synthesis of HNHDRG. The enzymes required for the synthesis of MHDRG, DHDRG, and THDRG are not known. In this study, we identified the glycosyltransferases UgtA (CDR20291_0008), which is required for the synthesis of all glycolipids, and UgtB (CDR20291_1186), which is required for the synthesis of DHDRG and THDRG. We propose a model where UgtA synthesizes only MHDRG, HexSDF synthesize HNHDRG from MHDRG, and UgtB synthesizes DHDRG and potentially THDRG from MHDRG. We also report that glycolipids are important for critical cell functions, including sporulation, cell size and morphology, maintaining membrane fluidity, colony morphology, and resistance to some membrane-targeting antimicrobials.
Importance: Clostridioides difficile infections are the leading cause of healthcare-associated diarrhea. C. difficile poses a risk to public health due to its ability to form spores and cause recurrent infections. Glycolipids make up ~50% of the polar lipids in the C. difficile membrane, a higher percentage than other common pathogens and include a unique glycolipid not present in other organisms. Here, we identify glycosyltransferases required for the synthesis of glycolipids in C. difficile and demonstrate the important role glycolipids play in C. difficile physiology.
Keywords: cell envelope; cell membrane; lipid synthesis; sporulation.
Conflict of interest statement
The authors declare no conflict of interest .
Figures


Update of
-
Identification of two glycosyltransferases required for synthesis of membrane glycolipids in Clostridioides difficile.bioRxiv [Preprint]. 2025 Jan 14:2025.01.14.632984. doi: 10.1101/2025.01.14.632984. bioRxiv. 2025. Update in: mBio. 2025 Mar 12;16(3):e0351224. doi: 10.1128/mbio.03512-24. PMID: 39868222 Free PMC article. Updated. Preprint.
Similar articles
-
Identification of two glycosyltransferases required for synthesis of membrane glycolipids in Clostridioides difficile.bioRxiv [Preprint]. 2025 Jan 14:2025.01.14.632984. doi: 10.1101/2025.01.14.632984. bioRxiv. 2025. Update in: mBio. 2025 Mar 12;16(3):e0351224. doi: 10.1128/mbio.03512-24. PMID: 39868222 Free PMC article. Updated. Preprint.
-
HexSDF Is Required for Synthesis of a Novel Glycolipid That Mediates Daptomycin and Bacitracin Resistance in C. difficile.mBio. 2023 Apr 25;14(2):e0339722. doi: 10.1128/mbio.03397-22. Epub 2023 Feb 14. mBio. 2023. PMID: 36786594 Free PMC article.
-
Identification of Clostridioides difficile mutants with increased daptomycin resistance.J Bacteriol. 2024 Mar 21;206(3):e0036823. doi: 10.1128/jb.00368-23. Epub 2024 Feb 20. J Bacteriol. 2024. PMID: 38376203 Free PMC article.
-
Regulatory networks: Linking toxin production and sporulation in Clostridioides difficile.Anaerobe. 2025 Feb;91:102920. doi: 10.1016/j.anaerobe.2024.102920. Epub 2024 Nov 7. Anaerobe. 2025. PMID: 39521117 Review.
-
Clostridioides difficile spore: coat assembly and formation.Emerg Microbes Infect. 2022 Dec;11(1):2340-2349. doi: 10.1080/22221751.2022.2119168. Emerg Microbes Infect. 2022. PMID: 36032037 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention (U.S) . 2019. Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.)
-
- Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Z Li J, Young VB. 2014. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5:3114. doi:10.1038/ncomms4114 - DOI - PMC - PubMed
-
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi:10.1056/NEJMoa1408913 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
