Rapid and sensitive isolation of Campylobacter jejuni using immunomagnetic separation from patient specimens exposed to oxygen
- PMID: 39964178
- PMCID: PMC11960043
- DOI: 10.1128/spectrum.01907-24
Rapid and sensitive isolation of Campylobacter jejuni using immunomagnetic separation from patient specimens exposed to oxygen
Abstract
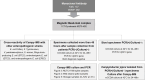
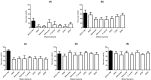
This study describes a method for detecting Campylobacter jejuni in patient stools with subsequent isolation using antibody-magnetic beads in conjunction with selective culture and PCR. Monoclonal antibodies specific for the flagellin A and major outer membrane protein of C. jejuni were generated; two clones (1C7 and 4B2) were used to coat magnetic beads for immunomagnetic separation (IMS). C. jejuni strain NCTC11168 was recovered from human stool samples spiked with varying concentrations (101-105 CFU/mL) by Campylobacter (Campy)-IMS or a conventional culture-based method and plated on modified charcoal-cefoperazone-deoxycholate agar; the number of colonies was enumerated. The detection limits of Campy-IMS and conventional culture-based method with spiked stool samples were 102 and 104 CFU/mL, respectively. The sensitivity of IMS-PCR was 10-10,000-fold higher than that of direct PCR. The recovery rate of C. jejuni from spiked stools stored for 12 to 72 h decreased from 72.3 to 5.9% with Campy-IMS and from 48.5 to 0.1% with the conventional culture-based method. Importantly, of 20 PCR (+)/bacterial culture (-) samples that were diagnosed as probable cases according to general criteria, 95% (19/20) were confirmed positive by Campy-IMS. Thus, this study suggests a solution to overcome the problems caused by the inconsistency between probable and confirmed cases of Campylobacter infection.
Importance: The isolation, cultivation, and maintenance of Campylobacter spp. are difficult because of the microaerophilic conditions and specific medium needed. Although selective media are useful for the initial isolation of Campylobacter, subsequent exposure of the sample to oxygen has a detrimental effect on the positive culture rate of Campylobacter, significantly lowering the isolation rate from patient samples. In this study, the detection limit was improved by combining immunomagnetic separation and PCR methods to quickly detect Campylobacter jejuni in clinical patient stool samples using antibody-magnetic beads. Therefore, this study is expected to improve confirmation of C. jejuni infection where diagnosis would previously fail with patient samples because of oxygen exposure, inappropriate diagnostic methods, and interference from other bacteria in the sample.
Keywords: Campylobacter jejuni; confirmed case; detection sensitivity; magnetic bead–antibody complex; monoclonal antibody; probable case; stool specimen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Development and evaluation of immunochromatographic assay for simple and rapid detection of Campylobacter jejuni and Campylobacter coli in human stool specimens.J Clin Microbiol. 2008 Apr;46(4):1226-31. doi: 10.1128/JCM.02170-07. Epub 2008 Feb 6. J Clin Microbiol. 2008. PMID: 18256225 Free PMC article.
-
Comparison of premier CAMPY enzyme immunoassay (EIA), ProSpecT Campylobacter EIA, and ImmunoCard STAT! CAMPY tests with culture for laboratory diagnosis of Campylobacter enteric infections.J Clin Microbiol. 2010 Nov;48(11):4022-7. doi: 10.1128/JCM.00486-10. Epub 2010 Sep 1. J Clin Microbiol. 2010. PMID: 20810765 Free PMC article.
-
Development and application of a real-time polymerase chain reaction method for Campylobacter jejuni detection.World J Gastroenterol. 2013 May 28;19(20):3090-5. doi: 10.3748/wjg.v19.i20.3090. World J Gastroenterol. 2013. PMID: 23716989 Free PMC article.
-
Multiplex PCR for identification of Campylobacter coli and Campylobacter jejuni from pure cultures and directly on stool samples.J Med Microbiol. 2005 Nov;54(Pt 11):1043-1047. doi: 10.1099/jmm.0.46203-0. J Med Microbiol. 2005. PMID: 16192435
-
Multiplex PCR for direct identification of Campylobacter spp. in human and chicken stools.J Med Microbiol. 2007 Oct;56(Pt 10):1350-1355. doi: 10.1099/jmm.0.47220-0. J Med Microbiol. 2007. PMID: 17893173
Cited by
-
Molecular diagnosis of campylobacter splenic abscess in an immunocompetent adolescent: A case report and literature review.IDCases. 2025 Jul 9;41:e02316. doi: 10.1016/j.idcr.2025.e02316. eCollection 2025. IDCases. 2025. PMID: 40726641 Free PMC article.
References
-
- Ministry of Food and Drug Safety . 2015. Occurrence of Foodborne Disease from 2003-2014. Available from: http://www.foodsafetykorea.go.kr./portal/healthyfoodlife/foodPosioningSt...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

