Ultrastructural Aspects of Corneal Functional Recovery in Rats Following Intrastromal Keratocyte Injection
- PMID: 39964324
- PMCID: PMC11838119
- DOI: 10.1167/iovs.66.2.45
Ultrastructural Aspects of Corneal Functional Recovery in Rats Following Intrastromal Keratocyte Injection
Abstract
Purpose: Donor tissue shortfalls and postsurgical complications are driving novel corneal tissue regeneration approaches. Corneal stromal keratocytes (CSKs) have shown promise in promoting corneal repair and restoring transparency. We investigated the impact of intrastromal CSK injection on corneal ultrastructure and proteoglycan (PG) distribution in a rat injury model.
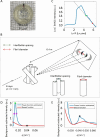
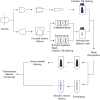
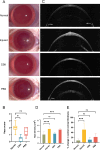
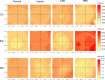
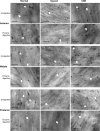
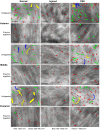
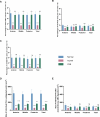
Methods: Rats were divided into four groups: normal (n = 12), injured (irregular phototherapeutic keratectomy centrally; n = 6), CSK (injured + human CSK intrastromal injection; n = 6), and PBS (injured + PBS injection; n = 6). Three weeks after treatment, corneas were examined by slit-lamp and optical coherence tomography. Corneal ultrastructure was analysed via small-angle x-ray scattering (collagen fibril diameter, interfibrillar spacing and matrix order), transmission electron microscopy with cuprolinic blue before and after chondroitinase digestion (CS/DS and KS PGs), and immunofluorescence staining (lumican and decorin).
Results: Irregular phototherapeutic keratectomy caused corneal opacity and significantly disrupted stromal ultrastructure, characterized by increased haze density (P < 0.0001), change in central corneal thickness (P = 0.0005), and interfibrillar spacing (P < 0.0001), along with decreased fibril diameter (P < 0.0001), matrix order (P < 0.0001), CS/DS (P < 0.0001) and KS (P < 0.0001) PGs, lumican, and decorin. CSK injection recovered corneal clarity and native stromal ultrastructure, with haze density (P = 0.8086), change in central corneal thickness (P = 0.9503), fibril diameter (P = 0.1139), interfibrillar spacing (P = 0.5879), matrix order (P = 0.9999), CS/DS (P = 0.9969) and KS (P = 0.2877) PGs, lumican, and decorin returning to normal. In contrast, the PBS group exhibited similar corneal injury responses to the injured group.
Conclusions: CSK injection resolved early stage corneal scarring by restoring stromal collagen arrangement and PG distribution, further endorsing its potential for treating corneal opacities.
Conflict of interest statement
Disclosure:
Figures
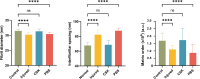


Similar articles
-
Safety and Feasibility of Intrastromal Injection of Cultivated Human Corneal Stromal Keratocytes as Cell-Based Therapy for Corneal Opacities.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3340-3354. doi: 10.1167/iovs.17-23575. Invest Ophthalmol Vis Sci. 2018. PMID: 30025076
-
Impact of keratocyte differentiation on corneal opacity resolution and visual function recovery in male rats.Nat Commun. 2024 Jun 11;15(1):4959. doi: 10.1038/s41467-024-49008-3. Nat Commun. 2024. PMID: 38862465 Free PMC article.
-
Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo.Exp Eye Res. 2022 Mar;216:108933. doi: 10.1016/j.exer.2022.108933. Epub 2022 Jan 11. Exp Eye Res. 2022. PMID: 35031282 Free PMC article.
-
The corneal fibrosis response to epithelial-stromal injury.Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review.
-
Keratocyte biology.Exp Eye Res. 2020 Jul;196:108062. doi: 10.1016/j.exer.2020.108062. Epub 2020 May 19. Exp Eye Res. 2020. PMID: 32442558 Review.
References
-
- Scott JE, Haigh M.. Identification of specific binding sites for keratan sulphate proteoglycans and chondroitin-dermatan sulphate proteoglycans on collagen fibrils in cornea by the use of cupromeronic blue in ‘critical-electrolyte-concentration’ techniques. Biochem J. 1988; 253: 607–610. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

