Clinical and Translational Results from PORTER, a Multicohort Phase I Platform Trial of Combination Immunotherapy in Metastatic Castration-Resistant Prostate Cancer
- PMID: 39964352
- PMCID: PMC11995007
- DOI: 10.1158/1078-0432.CCR-24-3693
Clinical and Translational Results from PORTER, a Multicohort Phase I Platform Trial of Combination Immunotherapy in Metastatic Castration-Resistant Prostate Cancer
Abstract
Purpose: Current immune checkpoint therapies offer limited benefits for metastatic castration-resistant prostate cancer. Novel combinations may enhance immunotherapy efficacy.
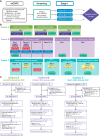
Patients and methods: We conducted an open-label, noncomparative platform trial (NCT03835533) in metastatic castration-resistant prostate cancer to assess nivolumab-based combinations. The cohorts were as follows: (A) bempegaldesleukin 0.006 mg/kg and nivolumab 360 mg i.v. every 3 weeks; (B) stereotactic body radiotherapy 30 to 50 Gy, CDX-301 75 μg/kg s.c. for 5 days, poly-ICLC 1 mg intramuscularly weekly twice for 3 weeks, and nivolumab 480 mg every 4 weeks; and (C) CDX-301 75 μg/kg for 10 days, INO-5151 3 mg intramuscularly on lead-in day 8, day 1 of cycles 1 to 3, and then every 12 weeks, and nivolumab 480 mg every 4 weeks. The primary endpoint was safety; secondary endpoints included composite response rate (radiographic, PSA, or circulating tumor cell responses), 6-month disease control rate, progression-free survival, and overall survival. Serial blood and tissue samples were analyzed for pharmacodynamics and association with disease control.
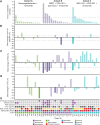
Results: A total of 43 patients were enrolled (n = 14, 15, and 14 in cohorts A, B, and C, respectively). Grade 3 to 4 treatment-related adverse events occurred in 10 (71%), 2 (13%), and 2 (14%) patients, respectively, with one grade 5 treatment-related adverse event in cohort A. Composite response rates were 7% (1/14), 33% (5/15), and 7% (1/14). Across cohorts, 6-month disease control was associated with preexisting memory/regulatory T cells, TNFα, and other inflammatory pathways.
Conclusions: Cohort B, which combined radiotherapy with CDX-301, poly-ICLC, and nivolumab, demonstrated encouraging clinical activity. Preexisting rather than treatment-induced immune activation was associated with clinical benefit across cohorts, highlighting the importance of baseline immune fitness.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
M.D. Galsky reports personal fees from Bristol Myers Squibb, Merck, Genentech, AstraZeneca, Pfizer, EMD Serono, Seagen, Janssen, Numab, Gilead Sciences, AbbVie, Fujifilm, and Daiichi Sankyo outside the submitted work. K.A. Autio reports grants from Pfizer, Janssen, Janux, Amgen, and Eli Lilly and personal fees from MashUp Media and IDEOlogy Health outside the submitted work. J.N. Graff reports grants from the Parker Institute for Cancer Immunotherapy during the conduct of the study. T.W. Friedlander reports grants from Johnson & Johnson Innovative Medicine and Genentech, grants and personal fees from Pfizer, and personal fees from Bristol Myers Squibb during the conduct of the study as well as grants and personal fees from Bicycle Therapeutics, Aadi Bioscience, Astellas Pharma, Gilead Sciences, MSD, and Samsungbioepis outside the submitted work. K.M. Shotts reports other support from Johnson & Johnson Innovative Medicine outside the submitted work. M. Spasic reports personal fees from Natera outside the submitted work. R.O. Chen reports personal fees and other support from Personalis during the conduct of the study as well as personal fees and other support from Personalis outside the submitted work. J. Skolnik reports other support from INOVIO during the conduct of the study. T. Keler reports employment with Celldex Therapeutics, Inc. and ownership of its stock. M.J. Yellin reports employment with Celldex Therapeutics, Inc. and ownership of its stock at the time the work was done. T.M. LaVallee reports grants from Bristol Myers Squibb and Cancer Research Institute during the conduct of the study as well as personal fees from Coherus BioSciences and LISCure Biosciences and other support from AstraZeneca outside the submitted work. J. Fairchild reports personal fees from the Parker Institute for Cancer Immunotherapy during the conduct of the study. U. Dugan reports grants from Bristol Myers Squibb during the conduct of the study as well as other support from Bristol Myers Squibb outside the submitted work. N. Bhardwaj reports grants from the Parker Institute for Cancer Immunotherapy outside the submitted work. S.K. Subudhi reports other support from Apricity Health LLC, Arcus Biosciences, Baird, Boxer Capital, Bristol Myers Squibb, Dendreon, DAVA Oncology, HERVolution, Johnson & Johnson, NoeticInsight, KAHR Medical Ltd, Kiniksa Pharmaceuticals, MacroGenics, Merck, Novartis, Portage, Regeneron, Rondo Therapeutics, The Clinical Comms Group, and AstraZeneca outside the submitted work. L. Fong reports grants from Bristol Myers Squibb and Nektar during the conduct of the study as well as grants from AbbVie, Amgen, Merck, and Bavarian Nordic; grants and personal fees from Roche/Genentech; personal fees from Innovent and Dendreon; and nonfinancial support from Actym, BioAtla, ImmunoGenesis, Nutcracker, RAPT, Senti, and TheraPaint outside the submitted work. No disclosures were reported by the other authors.
Figures


References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer Statistics, 2024. CA Cancer J Clin 2024;74:12–49. - PubMed
-
- Antonarakis ES, Park SH, Goh JC, Shin SJ, Lee JL, Mehra N, et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol 2023;41:3839–50. - PMC - PubMed
-
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 2017;35:40–7. - PubMed
-
- Graff JN, Burotto M, Fong PC, Pook D, Zurawski B, Manneh Kopp R, et al. 1771MO Pembrolizumab (pembro) plus enzalutamide (enza) for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): randomized double-blind phase III KEYNOTE-641 study. Ann Oncol 2023;34:S957.
-
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

