Crossovers are regulated by a conserved and disordered synaptonemal complex domain
- PMID: 39964475
- PMCID: PMC11833701
- DOI: 10.1093/nar/gkaf095
Crossovers are regulated by a conserved and disordered synaptonemal complex domain
Abstract
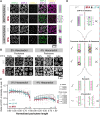
During meiosis, the number and distribution of crossovers (COs) must be precisely regulated through CO assurance and interference to prevent chromosome missegregation and genomic instability in the progeny. Here we show that this regulation of COs depends on a disordered and conserved domain within the synaptonemal complex (SC). This domain is located at the C-terminus of the central element protein SYP-4 in Caenorhabditis elegans. While not necessary for synapsis, the C-terminus of SYP-4 is crucial for both CO assurance and interference. Although the SYP-4 C-terminus contains many potential phosphorylation sites, we found that phosphorylation is not the primary regulator of CO events. Instead, we discovered that nine conserved phenylalanines are required to recruit a pro-CO factor predicted to be an E3 ligase and regulate the physical properties of the SC. We propose that this conserved and disordered domain plays a crucial role in maintaining the SC in a state that allows transmitting signals to regulate CO formation. While the underlying mechanisms remain to be fully understood, our findings align with existing models suggesting that the SC plays a critical role in determining the number and distribution of COs along chromosomes, thereby safeguarding the genome for future generations.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

