Addressing carbapenemase-producing extensively drug-resistant Pseudomonas aeruginosa: the potential of cefiderocol and ceftazidime/avibactam plus aztreonam therapy
- PMID: 39964628
- PMCID: PMC12062188
- DOI: 10.1007/s10096-025-05061-4
Addressing carbapenemase-producing extensively drug-resistant Pseudomonas aeruginosa: the potential of cefiderocol and ceftazidime/avibactam plus aztreonam therapy
Abstract
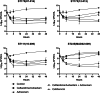
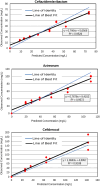
This study evaluated the activity of cefiderocol and the combination of ceftazidime/avibactam (CZA) plus aztreonam against carbapenemase-producing extensively drug-resistant (XDR) Pseudomonas aeruginosa isolates. Nine clinical XDR P. aeruginosa isolates with different sequence types and class A (GES) or B (VIM, IMP or NDM) carbapenemases were analysed. Time-kill assays assessed bacterial load reduction for each treatment, while chemostat experiments on four isolates validated these findings. All isolates showed resistance to CZA, with four also resistant to aztreonam. Seven isolates were susceptible to cefiderocol, but two displayed borderline susceptibility (MIC 2-4 mg/L). Time-kill assays demonstrated bactericidal activity by cefiderocol in six isolates at 24 h, while CZA plus aztreonam showed bactericidal effects in three isolates and synergistic/additive effects in four isolates. In the chemostat model, cefiderocol and CZA plus aztreonam were bactericidal in all four tested isolates, with cefiderocol showing greater bacterial reduction in three of these isolates. Both cefiderocol and CZA plus aztreonam achieved significant reductions in bacterial counts compared to controls, but there was no significant difference between cefiderocol monotherapy and the combination. Both cefiderocol and CZA plus aztreonam demonstrated activity against XDR P. aeruginosa carrying metallo-β-lactamase (MBL) and/or serine-β-lactamase (SBL) carbapenemases. Cefiderocol was the only consistently effective monotherapy with a bactericidal effect across all tested isolates in the chemostat model.
Keywords: Pseudomonas aeruginosa; Aztreonam; Cefiderocol; Ceftazidime/avibactam; Chemostat; PK/PD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Informed consent: Not applicable. Competing interests: MMM has received consulting fees and participated in educational activities from Pfizer, MSD, Shionogi, and Biomerieux. JPH has received consulting fees from Gilead, Tillots, Menarini and TFT Pharmaceuticals, and participated in educational activities from MSD, Pfizer and Angelini. A.O. has received fees as a speaker and/or research grants from MSD, Shionogi, Pfizer and Wockhardt. All other authors have no potential conflict of interest.
Figures
References
-
- Azam MW, Khan AU (2019) Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 1(24):350–9. 10.1016/j.drudis.2018.07.003 - PubMed
-
- Gellatly SL, Hancock REW (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173. 10.1111/2049-632X.12033 - PubMed
-
- Rodrigo-Troyano A, Melo V, Marcos PJ, Laserna E, Peiro M, Suarez-Cuartin G et al (2018) Pseudomonas aeruginosa in chronic obstructive pulmonary disease patients with frequent hospitalized exacerbations: a prospective multicentre study. Respiration 26:96:417–424. 10.1159/000490190 - PubMed
-
- del Barrio-Tofiño E, López-Causapé C, Oliver A (2020) Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int J Antimicrob Agents 56. 10.1016/j.ijantimicag.2020.106196 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous