Chlorhexidine vs Routine Foot Washing to Prevent Diabetic Foot Ulcers: A Randomized Clinical Trial
- PMID: 39964684
- PMCID: PMC11836759
- DOI: 10.1001/jamanetworkopen.2024.60087
Chlorhexidine vs Routine Foot Washing to Prevent Diabetic Foot Ulcers: A Randomized Clinical Trial
Erratum in
-
Error in Figure 3.JAMA Netw Open. 2025 Mar 3;8(3):e254939. doi: 10.1001/jamanetworkopen.2025.4939. JAMA Netw Open. 2025. PMID: 40067308 Free PMC article. No abstract available.
Abstract
Importance: Foot ulcers are a common and feared complication for people with diabetes because 20% of foot ulcers become infected and lead to a lower extremity amputation.
Objective: To evaluate the effect of daily foot care using chlorhexidine wipes vs soap-and-water wipes for 1 year on the risk of developing new foot complications in veterans with diabetes.
Design, setting, and participants: This double-blind, placebo-controlled, phase 2b randomized clinical trial was conducted at the Baltimore Veterans Affairs (VA) Medical Center between January 2019 to January 2023. Veterans were eligible if they had a diabetes diagnosis, were at high risk for diabetic foot complications, were ambulatory, had both feet, and did not have a current foot infection. Participants were randomly assigned 1:1 to receive either soap-and-water wipes (control group) or 2% chlorhexidine wipes (chlorhexidine group). Intention-to-treat data analysis was conducted from October 5, 2023, to April 24, 2024.
Intervention: Daily use of a 2% chlorhexidine wipe or a soap-and-water wipe on the feet for 1 year. Wipes were nearly identical in color, size, shape, thickness, feel, and scent. Both chlorhexidine and control groups received the same lotion for application on the feet after wipe use and education on foot self-care.
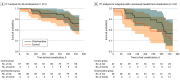
Main outcomes and measures: The primary outcome was time in days from randomization to new foot complication, including chronic foot ulcer, foot infection, or foot amputation.
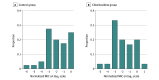
Results: A total of 175 participants (170 males [97%]; mean [SD] age at enrollment, 68 [9] years; 1 Asian [1%], 117 Black or African American [67%], 53 White [30%] individuals) were randomly assigned to the chlorhexidine group (n = 88) or the control group (n = 87). Twelve participants (14%) in the chlorhexidine group and 14 participants (16%) in the control group developed a new foot complication within 1 year. Median (IQR) time from randomization to development of a new foot complication was 232 (115-315) days. The reduction in hazard of new foot complications in the chlorhexidine group compared with the control group was not significant (hazard ratio, 0.83; 95% CI, 0.39-1.80). The intervention was well tolerated, with 145 participants (83%) continuing it over the study period. Sixty adverse events occurred, but none was related to the study products or procedures.
Conclusions and relevance: This randomized clinical trial found that daily use of chlorhexidine wipes for foot washing for 1 year did not lead to a significant reduction in the risk of new foot complications compared with daily use of soap-and-water wipes. The intervention was well tolerated, and the trial provides important lessons for future studies on diabetic foot ulcer prevention.
Trial registration: ClinicalTrials.gov Identifier: NCT03503370.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention . National diabetes statistics report 2020: estimates of diabetes and its burden in the United States. February 14, 2020. Accessed April 1, 2024. https://stacks.cdc.gov/view/cdc/85309/cdc_85309_DS1.pdf
-
- Margolis DJ, Malay DS, Hoffstad OJ, et al. . Incidence of diabetic foot ulcer and lower extremity amputation among Medicare beneficiaries, 2006 to 2008. Data Points #2. In: Data Points Publication Series. Agency for Healthcare Research and Quality; 2011. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

