ATP-sensitive potassium channels alter glycolytic flux to modulate cortical activity and sleep
- PMID: 39964713
- PMCID: PMC11874466
- DOI: 10.1073/pnas.2416578122
ATP-sensitive potassium channels alter glycolytic flux to modulate cortical activity and sleep
Abstract
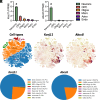
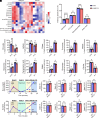
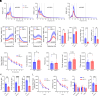
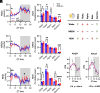
Metabolism plays a key role in the maintenance of sleep/wake states. Brain lactate fluctuations are a biomarker of sleep/wake transitions, where increased interstitial fluid (ISF) lactate levels are associated with wakefulness and decreased ISF lactate is required for sleep. ATP-sensitive potassium (KATP) channels couple glucose-lactate metabolism with excitability. Using mice lacking KATP channel activity (e.g., Kir6.2-/- mice), we explored how changes in glucose utilization affect cortical electroencephalography (EEG) activity and sleep/wake homeostasis. In the brain, Kir6.2-/- mice shunt glucose toward glycolysis, reducing neurotransmitter biosynthesis and dampening cortical EEG activity. Kir6.2-/- mice spent more time awake at the onset of the light period due to altered ISF lactate dynamics. Together, we show that Kir6.2-KATP channels act as metabolic sensors to gate arousal by maintaining the metabolic stability of sleep/wake states and providing the metabolic flexibility to transition between states.
Keywords: KATP channels; arousal; excitability; metabolism; sleep.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
Update of
-
Kir6.2-K ATP channels alter glycolytic flux to modulate cortical activity, arousal, and sleep-wake homeostasis.bioRxiv [Preprint]. 2024 Feb 28:2024.02.23.581817. doi: 10.1101/2024.02.23.581817. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 25;122(8):e2416578122. doi: 10.1073/pnas.2416578122. PMID: 38464274 Free PMC article. Updated. Preprint.
References
-
- Constantino N. J., et al. , Kir6.2-K (ATP) channels alter glycolytic flux to modulate cortical activity, arousal, and sleep-wake homeostasis. bioRxiv [Preprint] (2024). https://www.biorxiv.org/content/10.1101/2024.02.23.581817 (Accessed 28 February 2024). - DOI
MeSH terms
Substances
Grants and funding
- R01AG062550/HHS | NIH | National Institute on Aging (NIA)
- N/A/Alzheimer's Association (AA)
- R01 AG070830/AG/NIA NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 NS118558/NS/NINDS NIH HHS/United States
- R01AG080589/HHS | NIH | National Institute on Aging (NIA)
- R01 AG060056/AG/NIA NIH HHS/United States
- R01 AG080589/AG/NIA NIH HHS/United States
- R01 AG068330/AG/NIA NIH HHS/United States
- RF1 NS118558/NS/NINDS NIH HHS/United States
- P30GM127211/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- K01AG050719/HHS | NIH | National Institute on Aging (NIA)
- R01AG060056/HHS | NIH | National Institute on Aging (NIA)
- F31AG066302/HHS | NIH | National Institute on Aging (NIA)
- T32 NS115704/NS/NINDS NIH HHS/United States
- A20201775S/BrightFocus Foundation (BFF)
- R01 AG062550/AG/NIA NIH HHS/United States
- T32NS115704/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P20 GM148326/GM/NIGMS NIH HHS/United States
- P30 GM127211/GM/NIGMS NIH HHS/United States
- K01 AG050719/AG/NIA NIH HHS/United States
- R01AG068330/HHS | NIH | National Institute on Aging (NIA)
- P20GM148326/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- F31 AG066302/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

