Aberrant ERK signaling in astrocytes impairs learning and memory in RASopathy-associated BRAF mutant mouse models
- PMID: 39964758
- PMCID: PMC11996877
- DOI: 10.1172/JCI176631
Aberrant ERK signaling in astrocytes impairs learning and memory in RASopathy-associated BRAF mutant mouse models
Abstract
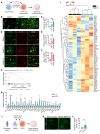
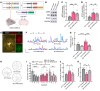
RAS/MAPK pathway mutations often induce RASopathies with overlapping features, such as craniofacial dysmorphology, cardiovascular defects, dermatologic abnormalities, and intellectual disabilities. Although B-Raf proto-oncogene (BRAF) mutations are associated with cardio-facio-cutaneous (CFC) syndrome and Noonan syndrome, it remains unclear how these mutations impair cognition. Here, we investigated the underlying neural mechanisms using several mouse models harboring a gain-of-function BRAF mutation (K499E) discovered in RASopathy patients. We found expressing BRAF K499E (KE) in neural stem cells under the control of a Nestin-Cre promoter (Nestin;BRAFKE/+) induced hippocampal memory deficits, but expressing it in excitatory or inhibitory neurons did not. BRAF KE expression in neural stem cells led to aberrant reactive astrogliosis, increased astrocytic Ca2+ fluctuations, and reduced hippocampal long-term depression (LTD) in mice. Consistently, 3D human cortical spheroids expressing BRAF KE also showed reactive astrogliosis. Astrocyte-specific adeno-associated virus-BRAF KE (AAV-BRAF KE) delivery induced memory deficits and reactive astrogliosis and increased astrocytic Ca2+ fluctuations. Notably, reducing extracellular signal-regulated kinase (ERK) activity in astrocytes rescued the memory deficits and altered astrocytic Ca2+ activity of Nestin;BRAFKE/+ mice. Furthermore, reducing astrocyte Ca2+ activity rescued the spatial memory impairments of BRAF KE-expressing mice. Our results demonstrate that ERK hyperactivity contributes to astrocyte dysfunction associated with Ca2+ dysregulation, leading to the memory deficits of BRAF-associated RASopathies.
Keywords: Development; Genetic diseases; Intellectual disability; Neurodevelopment; Neuroscience.
Figures





References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

